Significance
Polynucleotide ligases are an ancient superfamily of nucleic acid repair enzymes that join 3′-OH and 5′-PO4 DNA or RNA ends. Ligases react with ATP or NAD+ to form a covalent enzyme–adenylate intermediate in which AMP is linked via a P–N bond to a lysine side-chain. This paper reports crystal structures of a eukaryal ATP-dependent RNA ligase (Naegleria gruberi RNA ligase, NgrRnl) that illuminate the stereochemistry and two-metal catalytic mechanism of the lysine adenylylation reaction. A signature N-terminal domain of NgrRnl binds the ATP γ-phosphate and orients the pyrophosphate leaving group apical to the lysine nucleophile. NgrRnl is the founder of a distinct RNA ligase clade, with homologs in diverse bacterial, viral, and eukaryal proteomes.
Keywords: RNA repair, covalent nucleotidyltransferase, lysyl-AMP
Abstract
ATP-dependent RNA ligases are agents of RNA repair that join 3′-OH and 5′-PO4 RNA ends. Naegleria gruberi RNA ligase (NgrRnl) exemplifies a family of RNA nick-sealing enzymes found in bacteria, viruses, and eukarya. Crystal structures of NgrRnl at three discrete steps along the reaction pathway—covalent ligase-(lysyl-Nζ)–AMP•Mn2+ intermediate; ligase•ATP•(Mn2+)2 Michaelis complex; and ligase•Mn2+ complex—highlight a two-metal mechanism of nucleotidyl transfer, whereby (i) an enzyme-bound “catalytic” metal coordination complex lowers the pKa of the lysine nucleophile and stabilizes the transition state of the ATP α phosphate; and (ii) a second metal coordination complex bridges the β- and γ-phosphates. The NgrRnl N domain is a distinctively embellished oligonucleotide-binding (OB) fold that engages the γ-phosphate and associated metal complex and orients the pyrophosphate leaving group for in-line catalysis with stereochemical inversion at the AMP phosphate. The unique domain architecture of NgrRnl fortifies the theme that RNA ligases have evolved many times, and independently, by fusions of a shared nucleotidyltransferase domain to structurally diverse flanking modules. The mechanistic insights to lysine adenylylation gained from the NgrRnl structures are likely to apply broadly to the covalent nucleotidyltransferase superfamily of RNA ligases, DNA ligases, and RNA capping enzymes.
Biochemically diverse RNA repair systems rely on RNA ligases to maintain or manipulate RNA structure in response to purposeful RNA breakage events (1–5). RNA breaks destined for repair are inflicted by sequence-specific or structure-specific endoribonucleases during physiological RNA processing (e.g., tRNA splicing; kinetoplast mRNA editing) and under conditions of cellular stress (e.g., virus infection, starvation, unfolded protein response). RNA cleavage can occur either: (i) by a transesterification mechanism (generally metal-independent) that yields 2′,3′-cyclic-PO4 and 5′-OH ends; or (ii) via a hydrolytic mechanism (typically metal-dependent) that leaves 3′-OH and 5′-PO4 ends. RNA repair enzymes capable of sealing 2′,3′-cyclic-PO4/5′-OH breaks or 3′-OH/5′-PO4 breaks are distributed widely in all phylogenetic domains of life.
ATP-dependent RNA ligases join 3′-OH and 5′-PO4 RNA termini via a series of three nucleotidyl transfer steps similar to those of DNA ligases (6). In step 1, RNA ligase reacts with ATP to form a covalent ligase-(lysyl-Nζ)–AMP intermediate plus pyrophosphate. In step 2, AMP is transferred from ligase-adenylate to the 5′-PO4 RNA end to form an RNA–adenylate intermediate, AppRNA. In step 3, ligase catalyzes attack by an RNA 3′-OH on the RNA–adenylate to seal the two ends via a phosphodiester bond and release AMP.
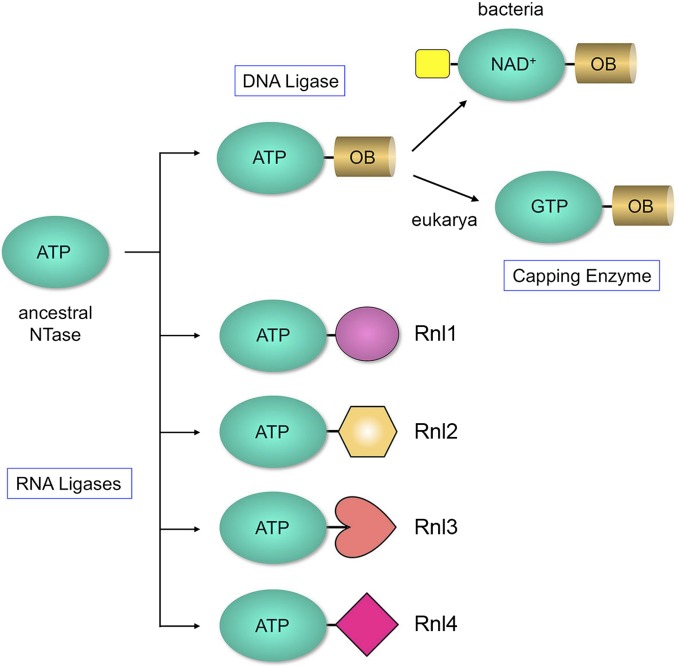
The autoadenylylation reaction of RNA ligases is performed by a nucleotidyltransferase (NTase) domain that is conserved in ATP-dependent and NAD+-dependent DNA ligases and GTP-dependent mRNA capping enzymes (6, 7). The NTase domain includes six peptide motifs (I, Ia, III, IIIa, IV, and V) that form the nucleotide-binding pocket (Fig. S1). Motif I contains the lysine that becomes covalently attached to the NMP. It is thought that modern RNA and DNA ligases and RNA capping enzymes evolved from an ancestral stand-alone ATP-using NTase domain via clade-specific acquisitions of flanking domain modules (7). DNA ligases and RNA capping enzymes have a shared core domain structure in which a conserved oligonucleotide-binding (OB)–fold domain is linked to the C terminus of the NTase domain (8–14). The scheme depicted in Fig. 1 posits that: (i) ATP-dependent DNA ligases evolved before divergence of bacteria, archaea, and eukarya; (ii) NAD+-dependent ligases evolved in bacteria by acquisition of a signature N-terminal module that confers NAD+ specificity (15, 16); and (iii) capping enzymes evolved uniquely in eukarya, via innovations within the NTase and OB domain that impart GTP specificity and recognition of diphosphate-terminated RNA ends.
Fig. S1.
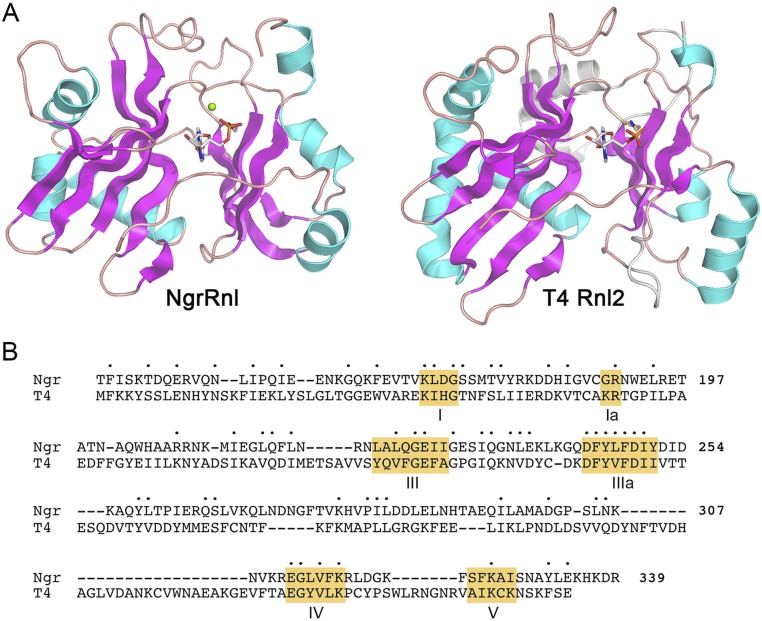
Comparision of the NTase domains of NgrRnl and T4 Rnl2. (A) The tertiary structures of the C-terminal NTase domain of NgrRnl–AMP•Mg2+ and the N-terminal NTase domain Rnl2•AMP were superimposed and offset horizontally. Shared structure elements are depicted in magenta for β-strands and cyan for α-helices. Elements unique to T4 Rnl2 are depicted in gray. (B) Structure-based alignment of the amino acid sequences of the NTase domains of NgrRnl and T4 Rnl2. Positions of side-chain identity/similarity are denoted by a dot (•). Gaps are indicated by a dash (–). The signature nucleotidyltransferase motifs are highlighted in gold boxes.
Fig. 1.
Scheme of ligase evolution. RNA ligases, DNA ligases, and mRNA capping enzymes comprise a superfamily of covalent nucleotidyltransferases that act via enzyme-(lysyl-Nζ)–NMP intermediates. They are thought to descend from an ancestral ATP-using NTase domain (turquois oval) by fusions to structurally diverse flanking domain modules (depicted in various shapes and colors), as discussed in the text.
In contrast, ATP-dependent RNA ligases appear to have evolved many times, and independently, by NTase fusions to structurally diverse C-terminal domain modules (Fig. 1). There are presently four structurally characterized RNA ligase families, exemplified by: (i) bacteriophage T4 RNA ligase 1 (Rnl1 family) (17); (ii) T4 RNA ligase 2 (Rnl2 family) (18); (iii) Pyrococccus abyssi RNA ligase (Rnl3 family) (19); and (iv) Clostridium thermocellum RNA ligase (Rnl4 family) (20, 21). The C-domain folds of these four RNA ligases are unrelated to one another and to the OB domains of DNA ligases or capping enzymes. The physiology and biochemistry of the Rnl families suggest a division of labor in RNA repair, whereby Rnl1 and Rnl4 are tailored to seal single-strand breaks in the loop of RNA stem-loops (22, 23), whereas Rnl2 is designed to seal 3′-OH/5′-PO4 nicks in duplex RNAs and RNA:DNA hybrids (24). The specificity of T4 Rnl1 for tRNA repair is conferred by its C domain (22). T4 Rnl2 depends on its C domain for binding and sealing a duplex RNA nick (7, 25). Our hypothesis is that there are more RNA ligase families to be discovered and that new flavors of RNA ligases will inform the biochemistry, biology, and evolutionary history of RNA repair.
Deinococcus radiodurans RNA ligase (DraRnl) and Naegleria gruberi RNA ligase (NgrRnl) represent a recently appreciated, though structurally uncharacterized, Rnl5 family of ATP-dependent RNA ligase (26–29). DraRnl and NgrRnl are template-directed RNA ligases capable of sealing nicked duplexes in which the 3′-OH strand is RNA. DraRnl and NgrRnl can join a RNAOH end to a 5′-pRNA or 5′-pDNA strand, but are unable to join when the 3′-OH strand is DNA. In this respect, they are similar to T4 Rnl2. However, unlike members of the four known RNA ligase families, DraRnl and NgrRnl lack a C-terminal appendage to their NTase domain. Instead, they contain a defining N-terminal domain that is important for overall 3′-OH/5′-PO4 nick sealing and ligase adenylylation (step 1), but dispensable for phosphodiester synthesis at a preadenylylated nick (step 3). DraRnl and NgrRnl prefer manganese as the metal cofactor for nick sealing, although either Mg2+ or Mn2+ can support the formation of the Rnl–AMP intermediate (26, 29). Manganese exerts profound effects on Deinococcus resistance to high-dose ionizing radiation and oxidative stress (30, 31). The gene encoding DraRnl is transiently up-regulated during recovery of Deinococcus from radiation exposure (32). It was speculated that DraRnl might contribute to the extreme radiation resistance of Deinococcus, by either repairing broken RNAs or by sealing broken DNAs that have acquired 3′-OH RNA termini by ribonucleotide addition (perhaps as a stop-gap measure during break and gap repair) (28).
Rnl5 homologs are encoded by 35 bacterial genera, representing 10 different phyla (28). Bacteriophages that prey on Aeromonas, Caulobacter, Mycobacterium, and Sinorhizobium also encode Rnl5 homologs. An archaeal homolog is present in Methanobrevibacter ruminantium. We were especially intrigued by the presence of Rnl5 homologs in many unicellular eukarya, including 13 genera of fungi, the amoebae Dictyostelium and Polysphondylium, and the amoebo-flagellate N. gruberi. (N. gruberi is an avirulent cousin of the human pathogen Naegleria fowleri, known as the “brain-eating amoeba,” which causes a devastating infection, primary amoebic meningoencephalitis.) We have purified and characterized recombinant NgrRnl, the exemplary eukaryal Rnl5 ligase (29). Here we report atomic structures of NgrRnl, captured at three discrete steps along the lysine–adenylylation reaction pathway. The structures suggest a two-metal mechanism of nucleotidyl transfer, in which a catalytic metal coordination complex lowers the pKa of the lysine nucleophile and stabilizes the transition state of the ATP α-phosphate, and a second metal coordination complex bridges the ATP β- and γ-phosphates. The NgrRnl N domain is a uniquely embellished OB-fold that binds the ATP γ-phosphate and associated metal complex and orients the pyrophosphate leaving group for in-line catalysis.
Results
Overview of the NgrRnl Structure.
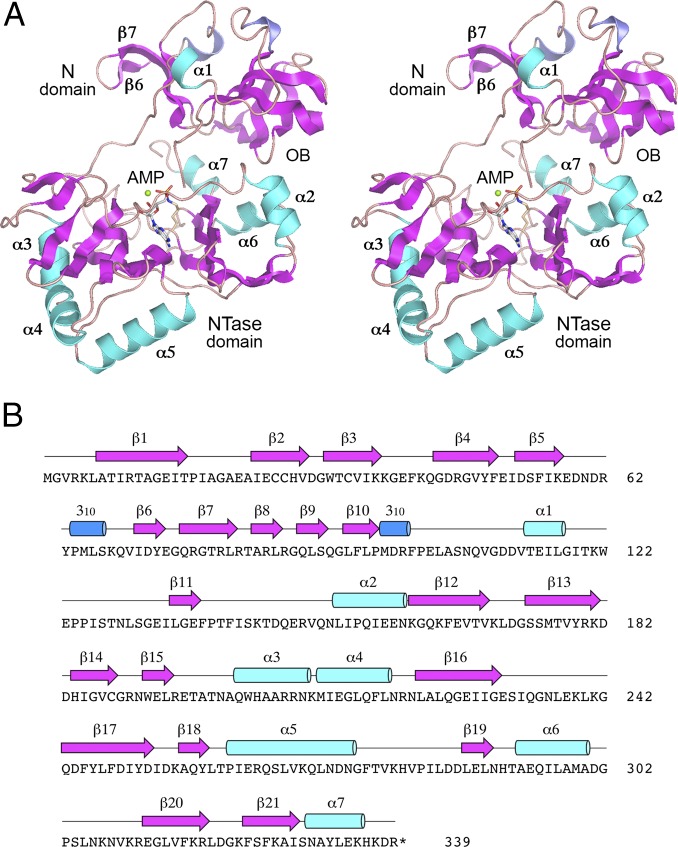
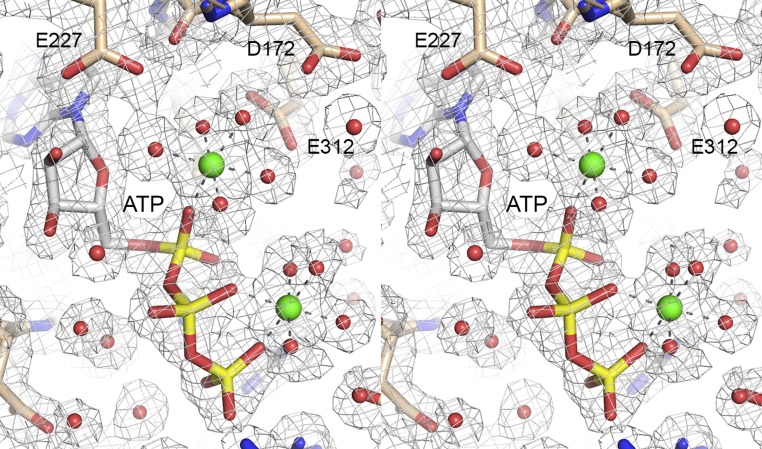
Crystallization of native and SeMet-substituted NgrRnl and structure determination of the native enzyme at 1.7 Å resolution are described in Methods and summarized in Table S1. A stereoview of the NgrRnl tertiary structure is shown in Fig. 2A. It is composed of 21 β-strands, seven α-helices, and two 310 helices, which are displayed over the amino acid sequence in Fig. 2B. NgrRnl consists of two domains. As expected, the C-terminal module (amino acids 123–339) is a classic NTase domain. A DALI search (33) of the PDB recovered the NTase domain of T4 Rnl2 as the top “hit,” with a z-score of 18.4 and 2.4 Å rmsd at 186 Cα positions (Table S2). The aligned tertiary structures of the NgrRnl and T4 Rnl2 NTase domains are shown in Fig. S1A. The primary structure alignment in Fig. S1B highlights the conservation of the signature NTase motifs. The NgrRnl NTase domain consists of two central antiparallel β-sheets, a six-strand sheet (with topology β15↓•β14↑•β13↓•β16↑•β17↓•β18↑) and a four-strand sheet (with topology β21↑•β20↓•β12↑•β19↓) that form the adenylate-binding pocket. Six α-helices decorate the lateral surfaces of the NTase domain. Electron density showed that AMP is covalently linked to the motif I Lys170 side-chain in the active site of the NTase domain (Fig. S2), consistent with our biochemical evidence that at least half of the recombinant NgrRnl protein was NgrRnl–AMP (29). The adenosine nucleotide is in the syn conformation. The AMP phosphate is coordinated by a divalent cation (green sphere), which we modeled as Mn2+ in light of the anomalous difference electron density overlying the metal atom (Fig. S2).
Table S1.
Crystallographic data and refinement statistics
| Data collection and statistics | SeMet-NgrRnl | NgrRnl–AMP | NgrRnl-K170M•ATP•Mn2+ | NgrRnl-K170M•Mn2+ | NgrRnl–AMP |
| Data collection | |||||
| Space group | P32 | P32 | P32 | P32 | P32 |
| Cell dimensions | |||||
| a, b, c (Å) | 55.3, 55.3, 102.7 | 55.3, 55.3, 102.6 | 55.0, 55.0, 100.3 | 55.5, 55.5, 98.6 | 55.1, 55.1, 100.7 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 |
| Resolution (Å) | 50–1.9 (1.95–1.9) | 50–1.7 (1.75–1.7) | 30–1.9 (1.95–1.9) | 30–2.2 (2.25–2.2) | 30–2.3 (2.35–2.3) |
| Wavelength (Å) | 0.9795 | 1.0000 | 0.9792 | 0.9792 | 1.7712 |
| Rpim | 0.028 (0.155) | 0.028 (0.220) | 0.055 (0.455) | 0.054 (0.240) | 0.069 (0.284) |
| CC(1/2) | 0.998 (0.914) | 0.998 (0.879) | 0.995 (0.723) | 0.991 (0.857) | 0.991 (0.759) |
| <I>/<σI> | 41.3 (3.8) | 31.9 (2.51) | 27.1 (3.0) | 22.1 (2.0) | 22.9 (3.3) |
| Completeness (%) | 97.7 (98.4) | 99.9 (99.7) | 100.0 (99.9) | 95.0 (84.6) | 98.5 (94.6) |
| Redundancy | 11.2 (8.4) | 4.9 (4.4) | 5.3 (5.1) | 2.3 (2.0) | 3.3 (2.5) |
| Unique reflections | 28,433 | 36,965 | 25,336 | 15,453 | 15,081 |
| Phasing | |||||
| No. of Se sites | 6 | ||||
| Figure of merit | 0.43 | ||||
| Refinement | |||||
| Rwork/Rfree | 0.167/0.209 | 0.179/0.247 | 0.190/0.266 | ||
| B-factors (Å2) Average/Wilson | 27.0/24.2 | 31.0/27.3 | 61.0/53.7 | ||
| RMSD | |||||
| Bond lengths (Å) | 0.022 | 0.019 | 0.012 | ||
| Bond angles (°) | 2.31 | 2.08 | 1.63 | ||
| Ramachandran plot | |||||
| Percent favored (%) | 97.3 | 97.6 | 95.8 | ||
| Percent allowed (%) | 2.7 | 2.4 | 4.2 | ||
| Outliers | None | None | None | ||
| Model contents | |||||
| Protomers/ASU | 1 | 1 | 1 | ||
| Protein residues | 340 | 339 | 339 | ||
| Water | 524 | 290 | 49 | ||
| Ligands/Ions | 1 AMP, 1 Mn2+ | 1 ATP, 2 Mn2+ | 1 Mn2+ | ||
| PDB ID | 5COT | 5COU | 5COV |
Values in parentheses refer to the highest resolution shell. Rfree set consists of 5% of data chosen randomly against which structures was not refined.
Fig. 2.
Overview of NgrRnl structure. (A) Stereoview of the tertiary structure of NgrRnl, which consists of an OB-containing N domain and a C-terminal NTase domain, depicted as a ribbon model with magenta β strands, cyan α helices, and blue 310 helices. The lysyl–AMP adduct in the active site is rendered as a stick model. Mn2+ is depicted as a green sphere. (B) Secondary structure elements (colored as in A) are displayed above the NgrRnl amino acid sequence.
Table S2.
Structural homologs of NgrRnl NTase domain
| Enzyme family | Source | PDB ID | Z-score | RMSD |
| ATP-dependent RNA ligase | Bacteriophage T4 Rnl2 | 2HVQ | 18.4 | 2.4 Å at 186 Cα positions |
| Trypanosoma brucei REL1 (Rnl2) | 1XDN | 16.6 | 2.4 Å at 185 Cα positions | |
| Aquifex aeolicus (Rnl3) | 3QWU | 15.5 | 3.4 Å at 188 Cα positions | |
| Pyrococcus abyssi (Rnl3) | 2VUG | 14.2 | 3.2 Å at 189 Cα positions | |
| Bacteriophage T4 Rnl1 | 2C5U | 14.2 | 3.4 Å at 182 Cα positions | |
| Clostridium thermocellum Pnkp-LIG (Rnl4) | 3TY8 | 10.8 | 3.9 Å at 188 Cα positions | |
| ATP-dependent DNA ligase | Mycobacterium tuberculosis LigD | 1VSO | 10.9 | 3.6 Å at 154 Cα positions |
| Chlorella virus PBCV1 | 2Q2T | 10.2 | 3.3 Å at 154 Cα positions | |
| NAD+-dependent DNA ligase | Thermus filiformis | 1V9P | 11.7 | 3.3 Å at 175 Cα positions |
| Enterococcus faecalis | 4LH6 | 11.5 | 3.4 Å at 174 Cα positions | |
| Escherichia coli | 2OWO | 11.0 | 3.5 Å at 175 Cα positions | |
| GTP-dependent RNA capping | Chlorella virus PBCV1 | 1CKN | 9.9 | 3.8 Å at 164 Cα positions |
| Mammalian | 3RTX | 9.5 | 3.6 Å at 157 Cα positions | |
| Candida albicans | 1P16 | 9.3 | 3.7 Å at 165 Cα positions |
Fig. S2.
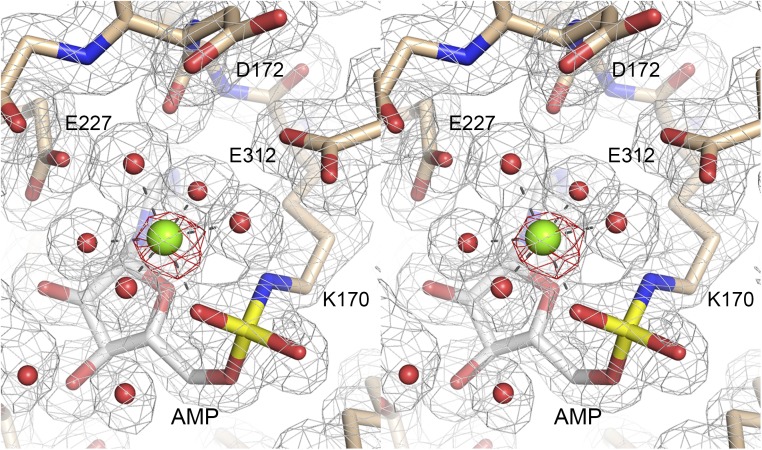
Stereoview of electron density for the lysyl–AMP adduct and catalytic metal ion. The 2Fo–Fc map contoured at 1σ is shown in gray mesh. The AMP (depicted as a stick model with gray carbons), metal ion (green sphere), and waters (red spheres) were omitted during refinement and phase calculation. NgrRnl amino acids are depicted as stick models with beige carbons. The AMP electron density is continuous with that of the Lys170 side chain. The anomalous difference map contoured at 5σ (from a dataset collected at 1.7712 Å wavelength) (Table S1) is depicted in red mesh. The atomic contacts of the octahedrally coordinated metal ion are indicated by dashed lines.
Adenine Nucleotide Specificity.
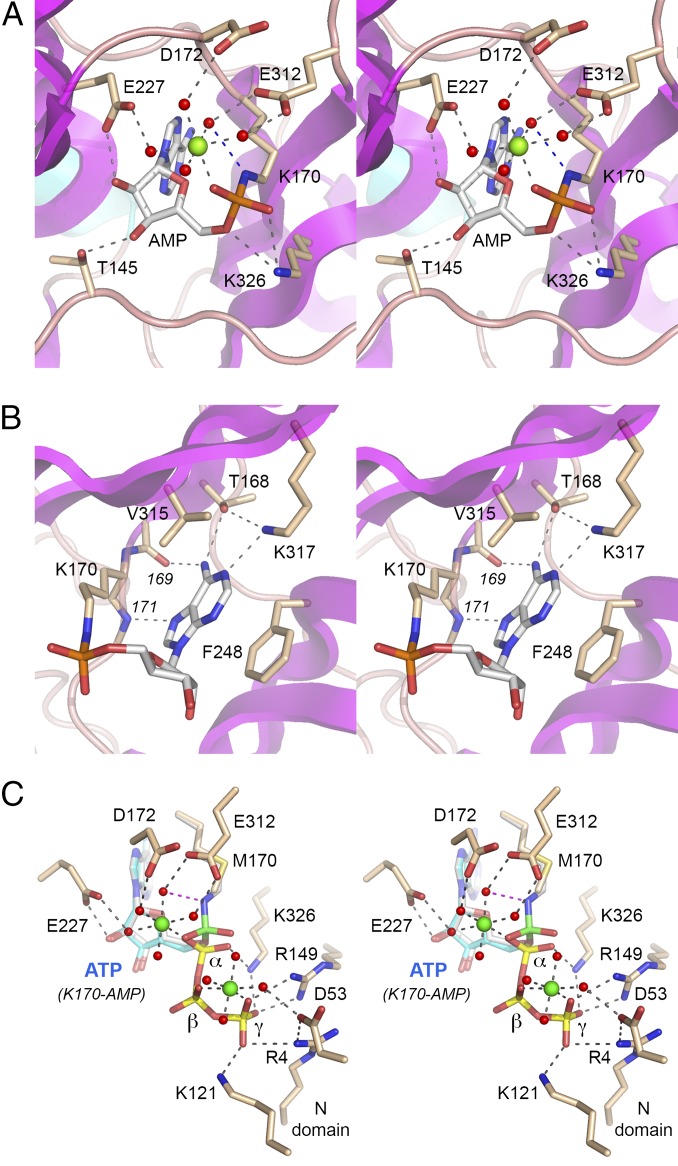
Enzymic contacts to the adenine base are highlighted in Fig. 3B. The purine ring is sandwiched between the aromatic ring of Phe248 and the hydrophobic side chain of Val315. Adenine specificity is conferred by amino acid side-chain hydrogen bonds from adenine-N6 to Thr168-Oγ and from Lys317-Nζ to adenine-N1. There are additional main-chain hydrogen bonds from adenine-N6 to Val169 carbonyl and from Leu171 amide to adenine-N7. The ribose 2′-OH and 3′-OH make hydrogen bonds to Glu227 and Thr145, respectively (Fig. 3A).
Fig. 3.
Structural insights to the mechanism of lysine adenylylation. (A and B) Stereo views of the active site of the NgrRnl-(lysyl)–AMP•Mn2+ intermediate in different orientations highlighting the Mn2+ coordination complex and enzymic contacts to the AMP ribose and phosphate (A) and contacts to the adenine nucleobase (B). Amino acids and AMP are shown as stick models with beige and gray carbons, respectively. A single Mn2+ ion and associated waters are depicted as green and red spheres, respectively. Atomic contacts are indicated by dashed lines. (C) Stereoview of the active site of the NgrRnl-K170M•ATP•(Mn2+)2 Michaelis complex. Amino acids and ATP are shown as stick models with beige and blue carbons, respectively; the ATP phosphorus atoms are colored yellow. Two Mn2+ ions and associated waters are depicted as green and red spheres, respectively. Atomic contacts are indicated by dashed lines. The superimposed lysyl–AMP adduct from the covalent intermediate structure is shown with gray carbons and a green α phosphorus atom (to highlight the stereochemical inversion of the phosphorus center after lysine adenylylation).
Key Role of the Metal Ion in Catalysis.
Waters occupy five of the ligand sites in the octahedral Mn2+ coordination complex in the NgrRnl–AMP structure (Fig. 3A). The ligase binds the Mn2+ complex via water-mediated contacts to Glu227, Glu312, and Asp172. [Glu227 and Glu312 are conserved in DraRnl as Glu230 and Glu305, respectively; their mutation to alanine in DraRnl effaced nick ligation and ligase adenylylation activity (27).] The sixth Mn2+ ligand site entails direct metal contact to one of the nonbridging AMP phosphate oxygens. The two other AMP phosphate oxygens are contacted by Lys326. The structure suggests that Lys326 and the metal stabilize a pentavalent transition state of the ATP α-phosphate during the lysine-adenylylation reaction. Our structure also reveals a likely role for the metal complex in stabilizing the unprotonated state of the lysine nucleophile before catalysis, via atomic contact of Lys170-Nζ to one of the waters in the metal complex (indicated by the blue dashed line in Fig. 3A).
The N Domain Is a Distinctively Embellished OB-Fold.
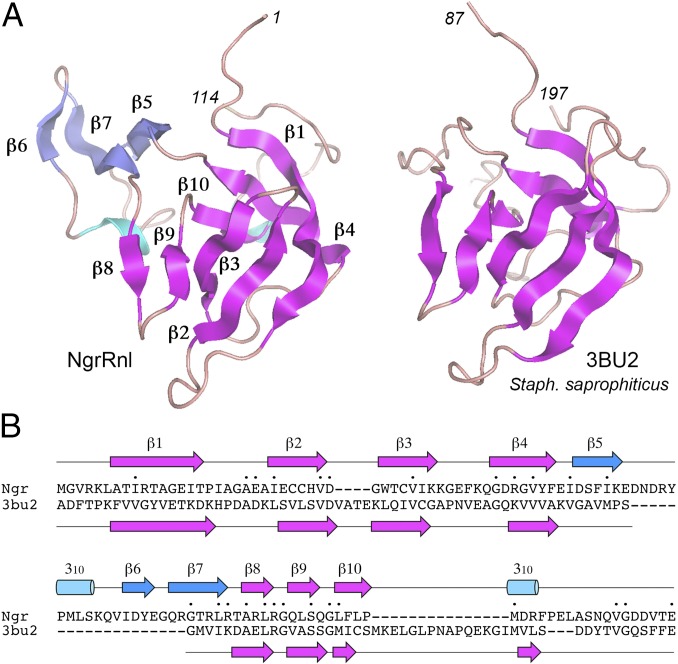
A DALI search of the NgrRnl N domain (amino acid 1–122) retrieved a C-terminal domain of a “putative tRNA binding protein” from Staphylococcus saprophyticus (PDB ID code 3BU2) as the top hit, with a z-score of 9.4 and 2.9 Å rmsd at 93 Cα positions. The aligned tertiary structures of the NgrRnl N-domain and 3BU2 are shown in Fig. 4A; the aligned secondary and primary structures are shown in Fig. 4B. The shared segment (formed by seven of the NgrRnl β-strands) is an OB-fold, an ancient domain found in many nucleic acid binding proteins. Indeed, the second best DALI hit against the NgrRnl N domain, with a z-score of 8.8, is an OB module found in Thermus thermophilus phenylalanyl-tRNA synthetase (34) (Table S3). NgrRnl’s OB-fold is embellished by a distinctive insert module (in blue in Fig. 4A), consisting of a three-strand antiparallel β-sheet (β6↑•β7↓•β5↑) and a 310 helix. To our knowledge, this is the first example of an RNA ligase that contains an OB domain and the first instance in which an OB domain is situated upstream of an NTase domain in a member of the covalent NTase superfamily. It is to be emphasized that the N-terminal NgrRnl domain has minimal structural similarity to the C-terminal OB-folds of DNA ligases and mRNA capping enzymes. For example, it aligns very weakly to the OB domains of Chlorella virus DNA ligase (9) (z-score 2.8; rmsd 6.5 Å at 60 Cα positions) and Chlorella virus mRNA capping enzyme (12) (z-score 2.1; rmsd 4.9 Å at 58 Cα positions). Moreover, there is virtually no amino acid identity between the NgrRnl N domain and the OB domains of DNA ligase and mRNA capping enzyme. This signifies to us that the N-terminal domain of the Rnl5 family of RNA ligases evolved separately from the C-terminal OB domains of DNA ligases and capping enzymes.
Fig. 4.
The N domain is a distinctively embellished OB-fold. (A) The tertiary structures of the NgrRnl N domain and the top DALI hit S. saprophyticius 3BU2 were aligned and then offset horizontally. The N- and C-terminal amino acid numbers are italicized. The NgrRnl secondary structure elements are labeled. The magenta β-strands are common to both proteins; the blue β-strands and the 310 helices are unique to NgrRnl. (B) The NgrRnl and 3BU2 primary structures are aligned according to DALI. Gaps in the alignment are indicated by dashes. Positions of amino acid side chain identity/similarity are indicated by a dot (•) above the sequence. The secondary structure elements of NgrRnl and 3BU2 (colored as in A) are shown above and below the respective amino acid sequences.
Table S3.
Structural homologs of NgrRnl N-domain
| Protein | Source | PDB ID | Z-score | RMSD |
| “tRNA binding” | Staphylococcus saprophyticus | 3BU2 | 9.4 | 2.9 Å at 93 Cα positions |
| Escherichia coli Trbp111 | 3ERS | 6.6 | 3.9 Å at 83 Cα positions | |
| Aminoacyl tRNA synthase | Thermus thermophilus PheRS | 1PYS | 8.9 | 2.9 Å at 89 Cα positions |
| Pyrococcus abyssi MetRS | 1MKH | 7.3 | 4.8 Å at 78 Cα positions | |
| DNA ligase | Chlorella virus PBCV1 (ATP) | IFVI | 2.8 | 6.5 Å at 60 Cα positions |
| Thermus filiformis (NAD+) | 1DGS | 2.3 | 4.5 Å at 61 Cα positions | |
| RNA capping | Candida albicans | 1P16 | 2.3 | 4.8 Å at 71 Cα positions |
| Chlorella virus PBCV1 | 1CKM | 2.1 | 4.9 Å at 58 Cα positions |
A Michaelis Complex of NgrRnl with ATP and Manganese.
To capture a mimetic of the Michaelis complex, we exploited a mutated version, K170M, in which the lysine nucleophile was replaced with methionine (effectively isosteric to lysine, minus the ε-amino group). NgrRnl-K170M was preincubated with 2 mM ATP and 5 mM MnCl2 before crystallization. The refined 1.9 Å structure (R/Rfree = 0.176/0.247) (Table S1) comprised the entire NgrRnl polypeptide, with electron density for ATP and two manganese ions in the active site (Fig. S3). The tertiary structure of the ligase protomer in the ATP complex was essentially identical to that of the ligase–AMP intermediate (z-score 51.0; rmsd of 0.6 Å at 339 Cα positions). Fig. 3C shows a stereoview of the active site of the ATP complex (ATP rendered with cyan carbons and yellow phosphorus atoms; Mn2+ as green spheres; water as red spheres), highlighting atomic interactions relevant to catalysis. For comparison, we included just the lysyl–AMP adduct from the NgrRnl–AMP structure (AMP with gray carbons and green Pα atom in Fig. 3C). The adenosine nucleosides superimpose almost perfectly and the Met170 side-chain is indeed virtually isosteric to Lys170. The salient mechanistic insights are summarized below.
Fig. S3.
Stereoview of electron density for ATP and two associated metal ions. The 2Fo–Fc map contoured at 1σ is shown in gray mesh. ATP (depicted as a stick model with gray carbons), metal ions (green spheres), and waters (red spheres) were omitted during refinement and phase calculation. NgrRnl amino acids are depicted as stick models with beige carbons. The atomic contacts of the octahedrally coordinated metal ions are indicated by dashed lines.
Geometry and Stereochemistry of Lysine Adenylylation.
The Lys170-Nζ is situated 2.9 Å from the ATP α phosphorus, in an apical orientation to the pyrophosphate leaving group (Nζ–Pα–O3α angle = 169°). Consistent with a single-step in-line mechanism, we see that the α-phosphate undergoes stereochemical inversion during the transition from ATP Michaelis complex to lysyl–AMP intermediate (Fig. 3C).
Two-Metal Mechanism of Nucleotidyl Transfer.
The “catalytic” octahedral Mn2+ complex (Mn1) that engages the ATP α-phosphate in the Michaelis complex is identical to that seen in the lysyl–AMP intermediate. The ATP-bound structure revealed a second Mn2+ atom (Mn2), coordinated octahedrally to four waters and to ATP β- and γ-phosphate oxygens. We infer that the second Mn2+ promotes lysine adenylylation by ensuring a proper conformation of the ATP triphosphate moiety conducive to expulsion of the pyrophosphate (PPi) leaving group.
Role of the N Domain in Orienting the PPi Leaving Group.
There are no direct enzymic contacts to the ATP β-phosphate. Lys326 and Arg149 are the only side chains of the NTase domain that engage the ATP phosphates, via a bifurcated interaction of Lys326 with α- and γ-phosphates and a bidentate interaction of Arg149 with the γ-phosphate. The structure highlights direct contacts to the ATP γ-phosphate from the N domain of NgrRnl, via Arg4 and Lys121, and to the second Mn2+ coordination complex via Asp53. The N domain contacts to ATP and Mn2 in the Michaelis complex aid in orienting the PPi leaving group during step 1 lysine adenylylation, and account for the severe decrement in step 1 activity when the N domain is deleted (29).
“Who’s on First”—Does Manganese Precede ATP?
Because the catalytic Mn2+ complex makes only one contact to the ATP α-phosphate, versus five ligand sites filled by waters, four of which are coordinated by NgrRnl active site side chains (Asp172, Glu227, Glu312), we hypothesize that the first metal cofactor Mn1 binds to the ligase before ATP. Because the second Mn2+ complex that bridges the β- and γ-phosphates makes only one water-mediated contact to the ligase (Asp53), and because a second metal is absent from the ligase-AMP intermediate (presumably having departed in a complex with the PPi leaving group), we envision that ATP•Mn(2) binds to ligase•Mn(1) to form a ligase•ATP•(Mn2+)2 Michaelis complex. To test the “Mn1 first” hypothesis, we grew crystals of NgrRnl-K170M that had been preincubated with 5 mM MnCl2 (without ATP), collected diffraction data to 2.2 Å resolution, and refined the structure to R/Rfree of 0.190/0.266 (Table S1). The difference density maps indicate that there is a single manganese ion, but no nucleotide, in the active site. The Mn2+ is coordinated to three waters, signifying that the metal coordination complex is incomplete in the absence of nucleotide.
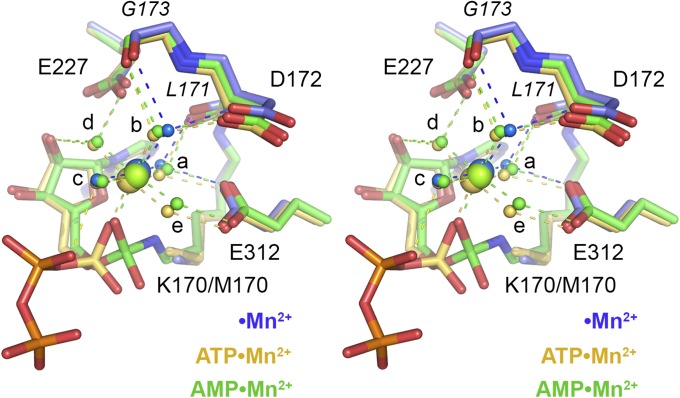
Fig. 5 shows a stereoview of the metals and metal ligands in the superimposed NgrRnl•Mn2+ (colored blue), NgrRnl•ATP•Mn2+ (colored gold), and NgrRnl–AMP•Mg2+ (colored green) structures. The five waters in the Mn2+ coordination complex of the adenylate-bound ligases are labeled “a” to “e.” The three water sites that are occupied in the NgrRnl•Mn2+ binary complex are “a,” “b,” and “c.” Water sites “d” and “e” that are vacant in the NgrRnl•Mn2+ binary complex occupy apical positions in the octahedron. Water “d” is coordinated jointly by Glu227-Oε and the adenosine ribose O2′, which might explain why this site is not filled before ATP binding. Two of the sites that are filled in the NgrRnl•Mn2+ binary complex make pairwise contacts with the ligase. Water “b” is engaged by hydrogen bonds to Asp172-Oδ and the Gly173 main-chain carbonyl. Water “a” (the one that contacts Lys170-Nζ) is engaged via hydrogen bonds to Glu312-Oε and the Leu171 main-chain carbonyl. We surmise that the initial binding of the catalytic metal Mn1 to the ligase stabilizes the unprotonated form of the lysine nucleophile in advance of ATP binding.
Fig. 5.
Comparison of the catalytic Mn2+ site with and without adenylate. Stereoview of the superimposed active sites of the NgrRnl-K170M•Mn2+ binary complex (with carbon atoms, waters, and Mn2+ colored blue), the NgrRnl-K170M•ATP•Mn2+ Michaelis complex (gold), and the NgrRnl-(lysyl)–AMP•Mn2+ intermediate (green). The five waters in the octahedral Mn2+ complex of the ATP and lysyl–AMP structures are labeled “a” to “e.” Only the “a,” “b,” and “c” water sites are occupied in the Mn2+ binary complex. Atomic contacts to the metal and waters are shown as dashed lines.
Discussion
The present study provides new insights to the structure, mechanism, and evolution of RNA ligases. We report the atomic structure of NgrRnl, the eukaryal founder of a Rnl5 family of nick sealing enzymes. The distinctive domain composition of NgrRnl fortifies the theme that multiple different clades of RNA ligases emerged by fusions of diverse accessory modules to a shared NTase domain. To our knowledge, NgrRnl is the first instance in which an OB domain is intrinsic to an RNA ligase polypeptide.
The NgrRnl•ATP•(Mn2+)2 structure, when superimposed on the NgrRnl–AMP•Mn2+ structure, captures (for the first time to our knowledge) a mimetic of the Michaelis complex of the ligase adenylylation reaction in which the nucleophile and PPi leaving group are oriented correctly for catalysis and the proper catalytic metal cofactor is coordinated in a mechanistically instructive fashion to ATP, the lysine nucleophile, and essential carboxylate side-chains of the NTase domain. Early structures of the covalent ligase–AMP intermediates of Chlorella virus DNA ligase (9) and Mycobacterium tuberculosis DNA ligase D (35) obtained from crystals that were soaked or grown in the presence of nonphysiological metals (e.g., lutetium or zinc) suggested the location of a “catalytic” metal near the AMP phosphate (corresponding to Mn1 in the NgrRnl structures). The structure of T4 Rnl1 with the unreactive analog AMPCPP in the active site obtained from crystals grown in the presence of calcium revealed a calcium ion in the catalytic metal site, coordinated to six waters and one AMPCPP α-phosphate oxygen (17). The closest counterpart to the present NgrRnl structures is that of the bacterial Rnl4 family RNA ligase–AMP•Mg2+ covalent intermediate formed in crystallo (21), in which a catalytic Mg2+ ion contacts the AMP phosphate directly and the remaining five positions in the octahedral metal complex are filled by waters, two of which are engaged by motif III and IV glutamate side chains (the equivalents of Glu227 and Glu312 in NgrRnl).
In addition to highlighting how direct metal interaction with the ATP α-phosphate would stabilize the transition state during lysine adenylylation, the NgrRnl structures offer clues to a longstanding conundrum in ligase biochemistry. As Robert Lehman pointed out in 1974 (36), it is a mystery how lysine (with a predicted pKa value of ∼10.5) loses its proton at physiological pH to attain the unprotonated state required for attack on the α-phosphorus of ATP or NAD+. In principle, ligase might use a general base to deprotonate the lysine. Alternatively, the active site milieu lowers the pKa of the lysine so that a significant fraction of the side-chain is unprotonated at physiological pH. Reduction of the lysine pKa could be driven by positive charge potential surrounding lysine-Nζ, analogous to how the pKa of the cysteine nucleophile in Yersinia protein tyrosine phosphatase is lowered to 4.7 by a network of positive potential from main-chain amides surrounding the sulfur (37, 38). Several crystal structures of ligase and capping enzyme absent metals (7, 12, 16, 19) provided scant support for either explanation. In these structures, the motif I lysine nucleophile is located next to the motif IV glutamate or aspartate side-chain (7, 12, 16, 19). The lysine and the motif IV carboxylate form an ion pair, the anticipated effect of which is to increase the pKa of lysine by virtue of surrounding negative charge. It is unlikely that a glutamate or aspartate anion could serve as general base to abstract a proton from the lysine cation. A potential solution to the problem would be if a divalent cation (coordinated by the motif IV carboxylate and the ATP α phosphate) abuts the lysine-Nζ and drives down its pKa.
An explicit model was proposed for the atomic contacts of the catalytic metal in the transition state of T4 DNA ligase (39), although there is no direct structural information on this enzyme in any state along its reaction pathway. According to the model, the catalytic metal makes direct contacts with lysine-Nζ (the nucleophile) and with the α–β bridging oxygen of ATP (the leaving group) as two of the component ligands of an octahedral metal coordination complex that also includes four waters. The model does not mandate a direct contact between the metal and nonbridging ATP α-phosphate oxygens; instead the nonbridging phosphate oxygens are depicted as accepting hydrogen bonds from two of the metal-bound waters (39). The NgrRnl structures presented here militate strenuously against such a model. Our structures implicate a metal-bound water, coordinated by the motif IV glutamate and situated within hydrogen-bonding distance of the lysine-Nζ, in stabilizing the unprotonated state of the lysine before ATP binding. Also, our structures are not consistent with the catalytic metal being involved in expulsion of the PPi leaving group, with which it makes no contacts.
Indeed, the NgrRnl structures instate a two-metal mechanism of lysine adenylylation, in which a second Mn2+ ion engages the ATP β- and γ-phosphates to attain a catalytically conducive orientation of the PPi leaving group apical to the lysine nucleophile. An earlier crystal structure of the isolated NTase domain of trypanosome RNA editing ligase 1 (TbrREL1, an Rnl2-family enzyme) in complex with ATP had revealed a single Mg2+ ion bridging the β- and γ-phosphates (40), analogous to the second metal in the NgrRnl•ATP structure. However, the TbrREL1 NTase•ATP structure is missing a catalytic metal ion that engages the α phosphate, and it represents an off-pathway state in which: (i) the noncatalytic Mg2+ ion occupies the position of the catalytic metal in the NgrRnl Michaelis complex; (ii) the ATP β- and γ-phosphates are in an unreactive orthogonal orientation to the lysine nucleophile (Nζ–Pα–O3α angle = 96°); and (iii) the motif IV glutamate forms a salt bridge with the lysine nucleophile that disfavors its deprotonation (Fig. S4).
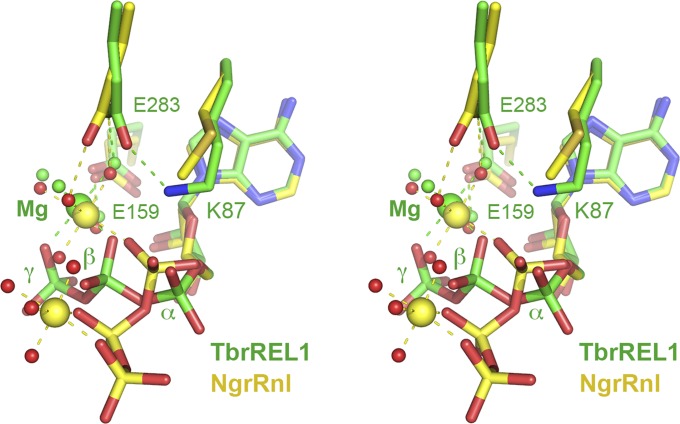
Fig. S4.
Comparison of NgrRnl•ATP•(Mn2+)2 and TbrREL1•ATP•Mg2+ structures. A stereoview of the superimposed structures is shown highlighting the ATPs and pertinent ligands. The metals are depicted as large yellow spheres in NgrRnl and a large green sphere in TbREL1. Waters are small red spheres in NgrRnl and small green spheres in TbREL1. Whereas the adenosine nucleosides adopt virtually identical conformations, there are striking differences in the positions and orientations of the β- and γ-phosphates (the PPi leaving group) and the metal coordination complexes that bridge the β- and γ-phosphates. The noncatalytic Mg2+ ion in TbrREL1 occupies the position of the catalytic metal in the NgrRnl Michaelis complex and it makes some of the same water-mediated enzymic contacts (e.g., to the motif III and motif IV glutamates). This positions the PPi leaving group orthogonal to the Lys87 nucleophile in TbREL1. This is clearly an off-pathway state. By contrast, in NgrRnl the leaving group is apical to the nucleophile.
In NgrRnl, the Mn2+-bound γ-phosphate makes electrostatic and hydrogen-bonding interactions with the signature N-terminal domain of NgrRnl, thereby explaining the importance of the N domain for ligase adenylylation via its role in binding ATP and orienting the PPi leaving group. [A second metal was seen previously in the T4 Rnl1•AMPCPP structure (17). Modeled as magnesium, it makes direct contact to just one of the AMPCPP β-phosphate oxygens; this Mg2+ ion is coordinated directly and via water to amino acids in the distinctive C-terminal domain of T4 Rnl1.]
Our findings highlight how Nature has adopted diverse structural solutions to the problem of leaving group geometry in the chemistry of lysine nucleotidylylation. In the case of NgrRnl and NAD+-dependent DNA ligases, the respective PPi and NMN leaving groups are oriented for in-line catalysis by clade-specific modules fused to the N terminus of the NTase domain: a unique OB domain in NgrRnl (29) and a unique “Ia domain” in NAD+-dependent DNA ligases (15, 16, 41). In the case of exemplary ATP-dependent DNA ligases and GTP-dependent RNA capping enzymes, the PPi leaving group is positioned by a conserved motif VI (RxDK) that defines a clade-specific OB module fused to the C terminus of the NTase domain (12, 42, 43).
Methods
NgrRnl and NgrRnl-K170M crystals were grown by hanging-drop vapor diffusion after mixing purified recombinant protein with 0.1 M Hepes pH 6.5, 30% PEG6000 precipitant solution. The crystals belonged to space group P32 and had one ligase protomer per asymmetric unit. The structure of native NgrRnl–AMP was solved using single-wavelength anomalous dispersion data from a single crystal of SeMet-NgrRnl. The final model of native NgrRnl–AMP was refined to Rwork/Rfree of 0.167/0.209 (Table S1). The NgrRnl structure was used as a starting model against which the diffraction data for single NgrRnl-K170M•ATP•Mn2+ and NgrRnl-K170M•Mn2+ crystals were refined (Table S1). Full details of protein purification, crystallization, diffraction data collection, and structure determination are provided in SI Methods.
SI Methods
Crystallization of NgrRnl.
Native wild-type NgrRnl and mutant NgrRnl-(K170M) were produced in Escherichia coli BL21(DE3) as His10Smt3•NgrRnl fusions and isolated from a soluble bacterial extract by Ni-affinity chromatography. After cleaving the tag during overnight dialysis in the presence of the Smt3 protease Ulp1, tag-free NgrRnl was recovered in the flow-through fraction during a second Ni-affinity step. NgrRnl was purified further by DEAE-Sephacel anion-exchange chromatography and Superdex-200 gel filtration as described previously (29). Selenomethionine (SeMet)-substituted NgrRnl was produced in E. coli B834 cells transformed with plasmid pET28b-His10Smt3-NgrRnl. A 4-L culture was grown at 37 °C in complete LeMaster medium containing 60 μg/mL kanamycin and 50 μg/mL racemic SeMet until A600 reached 0.6. The culture was then adjusted to 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubated for 3 h at 37 °C. SeMet-NgrRnl was purified from a soluble lysate of the IPTG-induced bacteria via the same protocol used for native NgrRnl (29).
Crystals of native NgrRnl and SeMet-NgrRnl were grown by hanging-drop vapor diffusion at room temperature after mixing on a coverslip 1 µL of protein solution (11.5 mg/mL) with 1 µL of reservoir solution containing 0.1 M Hepes pH 6.5, 30% PEG6000. A solution of NgrRnl-K170M (10.5 mg/mL) was adjusted either to 5 mM MnCl2 or to 2 mM ATP plus 5 mM MnCl2 and incubated for 15 min on ice before aliquots (1 µL) were mixed with 1 µL of 0.1 M Hepes, pH 6.5, 30% PEG6000. Crystals grew overnight. Single crystals were harvested, cryoprotected with parathone, and then flash-frozen in liquid nitrogen.
Diffraction Data Collection and Structure Determination.
Diffraction data were collected continuously from single crystals at the National Synchrotron Light Source beamline ×25 (for native NgrRnl to 1.7 Å resolution and for SeMet-NgrRnl to 1.9 Å resolution) and at the Advanced Photon Source beamline 24ID-C (for NgrRnl-K170M•ATP•Mn2+ to 1.9 Å resolution and for NgrRnl-K170M•Mn2+ to 2.2 Å resolution). All crystals belonged to space group P32 with similar unit cell dimensions (a and b = 55.0–55.5 Å; c = 98.6–102.6 Å), consistent with one protomer per asymmetric unit. Indexing and merging of the diffraction data were performed in HKL2000 (44). The structure of native NgrRnl was solved using single-wavelength anomalous dispersion data from a single crystal of SeMet-NgrRnl. Location of the heavy atom sites corresponding to SeMet residues, phasing, density modification, and initial model building were accomplished in Phenix (45). The difference maps showed density for AMP in the active site adjacent to and continuous with the Lys170 side chain, signifying that the covalent NgrRnl–AMP intermediate had been crystallized. The NgrRnl–AMP structure was iteratively refined in Refmac5 (46) and adjusted manually in O (47). A single metal ion was placed into electron density at the center of an octahedral coordination complex, with bond distances to the six metal ligands of 2.1–2.2 Å. The metal was modeled as manganese based on detection of an anomalous diffraction peak overlying the metal atom, as determined from datasets for native NgrRnl collected at wavelengths 1.5418 Å and 1.7712 Å. The final model of native NgrRnl–AMP, comprising the complete 339-aa NgrRnl polypeptide (plus an N-terminal serine left after cleavage of the Smt3 tag) was refined to Rwork/Rfree of 0.167/0.209 with no Ramachandran outliers and excellent geometry (Table S1).
The NgrRnl–AMP structure was used as a starting model against which the diffraction data for single NgrRnl-K170M•ATP•Mn2+ and NgrRnl-K170M•Mn2+ crystals were refined. The final models, which included the complete 339-aa NgrRnl-K170M polypeptide plus either ATP•(Mn2+)2 or a single Mn2+ ion, were refined to Rwork/Rfree of 0.179/0.247 and 0.190/0.266, respectively (Table S1).
Acknowledgments
This research was supported by National Institutes of Health Grant GM42498 (to S.S.) and Memorial Sloan Kettering Cancer Center Core Grant P30-CA008748.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5COT, 5COU, and 5COV).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516536112/-/DCSupplemental.
References
- 1.Amitsur M, Levitz R, Kaufmann G. Bacteriophage T4 anticodon nuclease, polynucleotide kinase and RNA ligase reprocess the host lysine tRNA. EMBO J. 1987;6(8):2499–2503. doi: 10.1002/j.1460-2075.1987.tb02532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87(3):405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 3.Schnaufer A, et al. An RNA ligase essential for RNA editing and survival of the bloodstream form of Trypanosoma brucei. Science. 2001;291(5511):2159–2162. doi: 10.1126/science.1058955. [DOI] [PubMed] [Google Scholar]
- 4.Schwer B, Sawaya R, Ho CK, Shuman S. Portability and fidelity of RNA-repair systems. Proc Natl Acad Sci USA. 2004;101(9):2788–2793. doi: 10.1073/pnas.0305859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandakumar J, Schwer B, Schaffrath R, Shuman S. RNA repair: An antidote to cytotoxic eukaryal RNA damage. Mol Cell. 2008;31(2):278–286. doi: 10.1016/j.molcel.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuman S, Lima CD. The polynucleotide ligase and RNA capping enzyme superfamily of covalent nucleotidyltransferases. Curr Opin Struct Biol. 2004;14(6):757–764. doi: 10.1016/j.sbi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Ho CK, Wang LK, Lima CD, Shuman S. Structure and mechanism of RNA ligase. Structure. 2004;12(2):327–339. doi: 10.1016/j.str.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Subramanya HS, Doherty AJ, Ashford SR, Wigley DB. Crystal structure of an ATP-dependent DNA ligase from bacteriophage T7. Cell. 1996;85(4):607–615. doi: 10.1016/s0092-8674(00)81260-x. [DOI] [PubMed] [Google Scholar]
- 9.Odell M, Sriskanda V, Shuman S, Nikolov DB. Crystal structure of eukaryotic DNA ligase-adenylate illuminates the mechanism of nick sensing and strand joining. Mol Cell. 2000;6(5):1183–1193. doi: 10.1016/s1097-2765(00)00115-5. [DOI] [PubMed] [Google Scholar]
- 10.Nandakumar J, Nair PA, Shuman S. Last stop on the road to repair: Structure of E. coli DNA ligase bound to nicked DNA-adenylate. Mol Cell. 2007;26(2):257–271. doi: 10.1016/j.molcel.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Nair PA, et al. Structural basis for nick recognition by a minimal pluripotent DNA ligase. Nat Struct Mol Biol. 2007;14(8):770–778. doi: 10.1038/nsmb1266. [DOI] [PubMed] [Google Scholar]
- 12.Håkansson K, Doherty AJ, Shuman S, Wigley DB. X-ray crystallography reveals a large conformational change during guanyl transfer by mRNA capping enzymes. Cell. 1997;89(4):545–553. doi: 10.1016/s0092-8674(00)80236-6. [DOI] [PubMed] [Google Scholar]
- 13.Fabrega C, Shen V, Shuman S, Lima CD. Structure of an mRNA capping enzyme bound to the phosphorylated carboxy-terminal domain of RNA polymerase II. Mol Cell. 2003;11(6):1549–1561. doi: 10.1016/s1097-2765(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 14.Doamekpor SK, Sanchez AM, Schwer B, Shuman S, Lima CD. How an mRNA capping enzyme reads distinct RNA polymerase II and Spt5 CTD phosphorylation codes. Genes Dev. 2014;28(12):1323–1336. doi: 10.1101/gad.242768.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sriskanda V, Shuman S. Conserved residues in domain Ia are required for the reaction of Escherichia coli DNA ligase with NAD+ J Biol Chem. 2002;277(12):9695–9700. doi: 10.1074/jbc.M111164200. [DOI] [PubMed] [Google Scholar]
- 16.Gajiwala KS, Pinko C. Structural rearrangement accompanying NAD+ synthesis within a bacterial DNA ligase crystal. Structure. 2004;12(8):1449–1459. doi: 10.1016/j.str.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 17.El Omari K, et al. Molecular architecture and ligand recognition determinants for T4 RNA ligase. J Biol Chem. 2006;281(3):1573–1579. doi: 10.1074/jbc.M509658200. [DOI] [PubMed] [Google Scholar]
- 18.Nandakumar J, Shuman S, Lima CD. RNA ligase structures reveal the basis for RNA specificity and conformational changes that drive ligation forward. Cell. 2006;127(1):71–84. doi: 10.1016/j.cell.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Brooks MA, et al. The structure of an archaeal homodimeric ligase which has RNA circularization activity. Protein Sci. 2008;17(8):1336–1345. doi: 10.1110/ps.035493.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith P, Wang LK, Nair PA, Shuman S. The adenylyltransferase domain of bacterial Pnkp defines a unique RNA ligase family. Proc Natl Acad Sci USA. 2012;109(7):2296–2301. doi: 10.1073/pnas.1116827109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, et al. Molecular basis of bacterial protein Hen1 activating the ligase activity of bacterial protein Pnkp for RNA repair. Proc Natl Acad Sci USA. 2012;109(33):13248–13253. doi: 10.1073/pnas.1209805109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang LK, Nandakumar J, Schwer B, Shuman S. The C-terminal domain of T4 RNA ligase 1 confers specificity for tRNA repair. RNA. 2007;13(8):1235–1244. doi: 10.1261/rna.591807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Chan CM, Wang P, Huang RH. Probing the substrate specificity of the bacterial Pnkp/Hen1 RNA repair system using synthetic RNAs. RNA. 2012;18(2):335–344. doi: 10.1261/rna.030502.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandakumar J, Ho CK, Lima CD, Shuman S. RNA substrate specificity and structure-guided mutational analysis of bacteriophage T4 RNA ligase 2. J Biol Chem. 2004;279(30):31337–31347. doi: 10.1074/jbc.M402394200. [DOI] [PubMed] [Google Scholar]
- 25.Nandakumar J, Shuman S. How an RNA ligase discriminates RNA versus DNA damage. Mol Cell. 2004;16(2):211–221. doi: 10.1016/j.molcel.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Martins A, Shuman S. An RNA ligase from Deinococcus radiodurans. J Biol Chem. 2004;279(49):50654–50661. doi: 10.1074/jbc.M407657200. [DOI] [PubMed] [Google Scholar]
- 27.Raymond A, Shuman S. Deinococcus radiodurans RNA ligase exemplifies a novel ligase clade with a distinctive N-terminal module that is important for 5′-PO4 nick sealing and ligase adenylylation but dispensable for phosphodiester formation at an adenylylated nick. Nucleic Acids Res. 2007;35(3):839–849. doi: 10.1093/nar/gkl1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmier BJ, Shuman S. Effects of 3′-OH and 5′-PO4 base mispairs and damaged base lesions on the fidelity of nick sealing by Deinococcus radiodurans RNA ligase. J Bacteriol. 2014;196(9):1704–1712. doi: 10.1128/JB.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unciuleac MC, Shuman S. Characterization of a novel eukaryal nick-sealing RNA ligase from Naegleria gruberi. RNA. 2015;21(5):824–832. doi: 10.1261/rna.049197.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7(3):237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 31.Slade D, Radman M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev. 2011;75(1):133–191. doi: 10.1128/MMBR.00015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, et al. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc Natl Acad Sci USA. 2003;100(7):4191–4196. doi: 10.1073/pnas.0630387100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holm L, Kääriäinen S, Rosenström P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24(23):2780–2781. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosyak L, Reshetnikova L, Goldgur Y, Delarue M, Safro MG. Structure of phenylalanyl-tRNA synthetase from Thermus thermophilus. Nat Struct Biol. 1995;2(7):537–547. doi: 10.1038/nsb0795-537. [DOI] [PubMed] [Google Scholar]
- 35.Akey D, et al. Crystal structure and nonhomologous end-joining function of the ligase component of Mycobacterium DNA ligase D. J Biol Chem. 2006;281(19):13412–13423. doi: 10.1074/jbc.M513550200. [DOI] [PubMed] [Google Scholar]
- 36.Lehman IR. DNA ligase: Structure, mechanism, and function. Science. 1974;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- 37.Zhang ZY, Dixon JE. Active site labeling of the Yersinia protein tyrosine phosphatase: The determination of the pKa of the active site cysteine and the function of the conserved histidine 402. Biochemistry. 1993;32(36):9340–9345. doi: 10.1021/bi00087a012. [DOI] [PubMed] [Google Scholar]
- 38.Stuckey JA, et al. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 A and the complex with tungstate. Nature. 1994;370(6490):571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- 39.Cherepanov AV, de Vries S. Kinetic mechanism of the Mg2+-dependent nucleotidyl transfer catalyzed by T4 DNA and RNA ligases. J Biol Chem. 2002;277(3):1695–1704. doi: 10.1074/jbc.M109616200. [DOI] [PubMed] [Google Scholar]
- 40.Deng J, Schnaufer A, Salavati R, Stuart KD, Hol WG. High resolution crystal structure of a key editosome enzyme from Trypanosoma brucei: RNA editing ligase 1. J Mol Biol. 2004;343(3):601–613. doi: 10.1016/j.jmb.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 41.Sriskanda V, Moyer RW, Shuman S. NAD+-dependent DNA ligase encoded by a eukaryotic virus. J Biol Chem. 2001;276(39):36100–36109. doi: 10.1074/jbc.M105643200. [DOI] [PubMed] [Google Scholar]
- 42.Sawaya R, Shuman S. Mutational analysis of the guanylyltransferase component of mammalian mRNA capping enzyme. Biochemistry. 2003;42(27):8240–8249. doi: 10.1021/bi034396d. [DOI] [PubMed] [Google Scholar]
- 43.Samai P, Shuman S. Kinetic analysis of DNA strand joining by Chlorella virus DNA ligase and the role of nucleotidyltransferase motif VI in ligase adenylylation. J Biol Chem. 2012;287(34):28609–28618. doi: 10.1074/jbc.M112.380428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 45.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 47.Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]