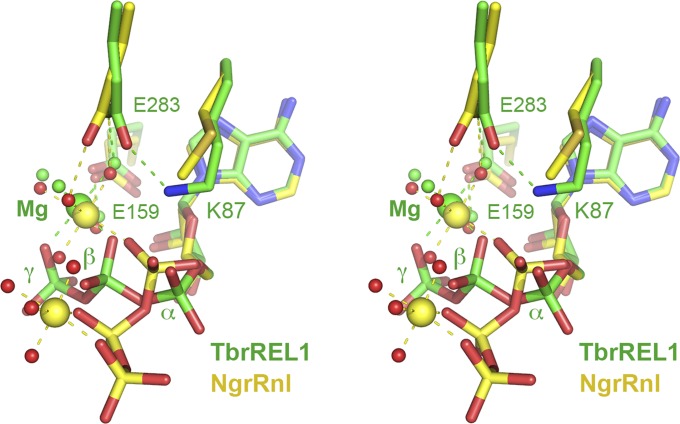
Fig. S4.
Comparison of NgrRnl•ATP•(Mn2+)2 and TbrREL1•ATP•Mg2+ structures. A stereoview of the superimposed structures is shown highlighting the ATPs and pertinent ligands. The metals are depicted as large yellow spheres in NgrRnl and a large green sphere in TbREL1. Waters are small red spheres in NgrRnl and small green spheres in TbREL1. Whereas the adenosine nucleosides adopt virtually identical conformations, there are striking differences in the positions and orientations of the β- and γ-phosphates (the PPi leaving group) and the metal coordination complexes that bridge the β- and γ-phosphates. The noncatalytic Mg2+ ion in TbrREL1 occupies the position of the catalytic metal in the NgrRnl Michaelis complex and it makes some of the same water-mediated enzymic contacts (e.g., to the motif III and motif IV glutamates). This positions the PPi leaving group orthogonal to the Lys87 nucleophile in TbREL1. This is clearly an off-pathway state. By contrast, in NgrRnl the leaving group is apical to the nucleophile.