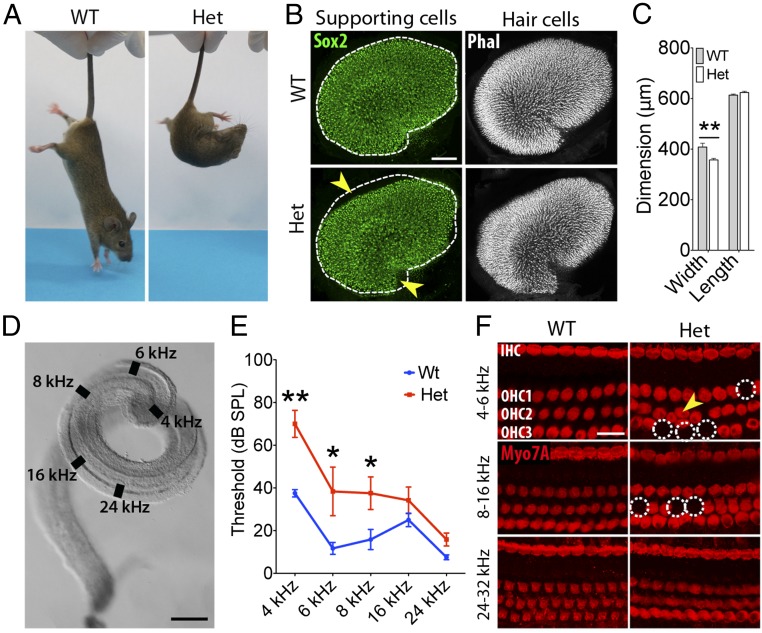
Fig. 3.
Vestibular dysfunction and deafness in heterozygous SoxC CKO mice. (A) In the tail-suspension test, a 4-wk-old WT mouse reaches downward for the substrate, whereas a heterozygous SoxC CKO animal (Het) displays ataxic curling. (B) In utricles from 4-wk-old WT and Het animals, supporting cells are immunolabeled with anti-Sox2 (green), and hair bundles are revealed by phalloidin labeling (white). Fitting the outline of the WT macula (dashed line) to the mutant's macula demonstrates a decrease in the organ’s width, which was quantified by measuring the distance across the organ at the notch on its neural edge and perpendicular to its long axis. (Scale bar, 100 μm.) (C) Measurements confirm that the difference observed in B is statistically significant and corresponds to a 13% decrease in the organ’s width (**P = 0.0076) (WT n = 5, Het n = 6). (D) A dissected murine cochlear duct shows the anatomical positions of the hair cells most sensitive to the frequencies at which auditory brainstem responses were measured. (Scale bar, 250 μm.) (E) Heterozygous SoxC CKO mice (n = 6) display a significant elevation in their thresholds for auditory brainstem responses with respect to WT animals (n = 6). *P < 0.05, **P < 0.001. (F) Immunolabeling of a WT ear for Myo7A (red) reveals a regular hair cell array with one row of inner hair cells (IHC) and three rows of outer hair cells (OHC1, OHC2, and OHC3) in the apical (4–6 kHz and 8–16 kHz) and middle (16–24 kHz) cochlear turns. In a heterozygous SoxC CKO animal, in contrast, some hair cells in the apical turn are missing (dotted circles), and others are misaligned (yellow arrowhead). (Scale bar, 25 μm.)