Abstract
Background
The WHO classified pancreatic neuroendocrine neoplasms in 2010 as G1, G2, and neuroendocrine carcinoma (NEC), according to the Ki67 labeling index (LI). However, the clinical behavior of NEC is still not fully studied. We aimed to clarify the clinicopathological and molecular characteristics of NECs.
Methods
We retrospectively evaluated the clinicopathological characteristics, KRAS mutation status, treatment response, and the overall survival of eleven pNEC patients diagnosed between 2001 and 2014 according to the WHO 2010. We subclassified WHO-NECs into well-differentiated NEC (WDNEC) and poorly differentiated NEC (PDNEC). The latter was further subdivided into large-cell and small-cell subtypes.
Results
The median Ki67 LI was 69.1 % (range 40–95 %). Eleven WHO-NECs were subclassified into 4 WDNECs and 7 PDNECs. The latter was further separated into 3 large-cell and 4 small-cell subtypes. Comparisons of WDNEC vs. PDNEC revealed the following traits: hypervascularity on CT, 50 % (2/4) vs. 0 % (0/7) (P = 0.109); median Ki67 LI, 46.3 % (40–53 %) vs. 85 % (54–95 %) (P = 0.001); Rb immunopositivity, 100 % (4/4) vs. 14 % (1/7) (P = 0.015); KRAS mutations, 0 % (0/4) vs. 86 % (6/7) (P = 0.015); response rates to platinum-based chemotherapy, 0 % (0/2) vs. 100 % (4/4) (P = 0.067), and median survival, 227 vs. 186 days (P = 0.227).
Conclusions
The WHO-NEC category may be composed of heterogeneous disease entities, namely WDNEC and PDNEC. These subgroups tended to exhibit differing profiles of Ki67 LI, Rb immunopositivity and KRAS mutation, and distinct response to chemotherapy. Further studies for the reevaluation of the current WHO 2010 classification are warranted.
Electronic supplementary material
The online version of this article (doi:10.1007/s00535-014-0987-2) contains supplementary material, which is available to authorized users.
Keywords: Neuroendocrine carcinoma, Ki67 labeling index, KRAS mutation, WHO classification
Introduction
Ki67 is a powerful prognostic marker of pancreatic neuroendocrine neoplasms (pNENs) [1] and, accordingly, the remarkable revision was made from the former 2000 World Health Organization (WHO) classification system to the current WHO 2010 terminology system, in which mitotic count and/or Ki67 labeling index (LI) were adopted as the pivotal indicator of stratification [2]. NENs are now to be categorized into neuroendocrine tumor (NET)-G1, NET-G2, and neuroendocrine carcinoma (NEC). Whereas NETs-G1/G2 are invariably composed of tumor cells with well-differentiated morphology, NECs usually have poorly differentiated histology with Ki67 LI > 20 % [2, 3]. Accordingly, all NENs with Ki67 LI > 20 % are defined as NEC. Clinically, these tumors are treated with the same platinum-based chemotherapy regimens as small-cell lung cancers [4–6]. However, some reports have recently indicated that a proportion of well-differentiated NENs might have proliferative rates above the threshold for NET-G2 [7, 8]. In addition, the Nordic NEC study reported that patients with a Ki67 <55 % had low responses to platinum-based chemotherapy [9]. We suppose that the current NEC category, as defined by the WHO 2010 classification (WHO-NEC), includes two groups that differ in clinical behaviors as well as pathological characteristics. Information about the clinicopathological features of WHO-NEC group is scant [7–10]. Therefore, we aimed to further characterize the WHO-NEC group in terms of pathological findings, molecular characteristics, and clinical behaviors.
Patients and methods
Patients
We retrospectively retrieved all of the pNENs diagnosed between January 2001 and March 2014 from our hospital database. All patients were recategorized as NET-G1, NET-G2, or NEC according to the WHO 2010 classification. Specimens for histological examination were obtained from preoperative endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), biopsy, and/or surgical resection. All patients diagnosed with small-cell carcinoma were subsequently assessed by contrast enhanced (CE) chest MDCT to exclude the possibility of metastasis from a primary lung cancer [11]. This study was approved by our institutional review board.
Diagnostic and prognostic characterization
The following features were recorded for all patients: age, gender, symptoms, hormonal syndromes, primary and metastatic locations, European Neuroendocrine Tumor Society (ENETS) TNM stage [12], and CE-MDCT features such as anatomical location, tumor size, and contrast enhancement. We recorded the details of all treatments administered to the patients, particularly platinum-based chemotherapy [4, 5, 13].
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and sample preparation
EUS-FNA procedures were performed using a convex linear-array echoendoscope (GF-UGT240 or GF-UCT260; Olympus Optical Co Ltd, Tokyo, Japan) paired with an ultrasound machine (SSD5500 or Prosound α10; Aloka, Tokyo, Japan). We used 22-gauge needles (NA-11J-KBor NA-200H-8022; Olympus Medical System Corp. Ltd., Tokyo, Japan or EchoTip-Ultra Needle; Cook Endoscopy Inc., Winston Salem, N.C., USA or Expect; Boston Scientific Japan, Tokyo, Japan).
Aspirated materials were divided for cytopathological evaluation, cell-block preparation, and KRAS mutation analysis. In all patients, specimen adequacy was evaluated on-site by Diff Quick staining (Diff-Quik; Kokusai Shiyaku, Kobe, Japan) by a cytopathologist or cytotechnologist. Cell-blocks were prepared after the fresh specimens were immediately fixed in 10 % formalin and embedded in paraffin. Sliced sections then were stained by hematoxylin and eosin, as well as by immunohistochemical staining (IHC) [14].
Histological evaluation
We defined tumors as NEC that showed diffuse expression of neuroendocrine markers and Ki67 LI of more than 20 %. In accordance with the 2010 WHO classification, tumors characterized by high-grade cytological atypia, apparent pleomorphism, extensive necrosis, and prominent mitotic activity were categorized into poorly differentiated NEC (PDNEC). Of PDNECs, tumors characterized by diffuse growth of highly atypical cells with small-sized to medium-sized nuclei, finely granular chromatin, and inconspicuous nucleoli, were categorized as small-cell NEC (SCNEC). Carcinomas with large nuclei, coarse chromatin and well-visible nucleoli with nested proliferation were categorized as large-cell NEC (LCNEC). Furthermore, we attempted to extract those tumors whose cytological features were blander than that of PDNEC and rather similar to NET-G2; that is, tumors composed predominantly of cells with low nucleocytoplasmic ratio and small-sized to medium-sized, ovoid nuclei, growing with minimal pleomorphism, and lacking extensive necrosis. We designated these tumors as ‘well differentiated NEC (WDNEC)’, and separated them from SCNECs and LCNECs. All slides were reviewed and reclassified by the same pathologist (WH).
Immunohistochemistry and Ki67 labeling index
IHC was performed using monoclonal antibodies for chromogranin A (clone SP12, rabbit, 1:200, Neo Markers), synaptophysin (clone SP11, rabbit, 1:100, Neo Markers, Fremont, CA, USA), Ki67 (clone SP6, rabbit, 1:200; Neo Markers), and Rb (clone 3H9, mouse, 1:300; MBL).
The measurement of Ki67 LI was performed under the assistance of digital pathology technology. Briefly, slides were digitally scanned using a Scan Scope XT (Aperio Technologies, Vista, CA, USA). All sections were reviewed to exclude portions with extensive desmoplasia, necrosis and regions with bleeding. The ultimate Ki67 LI was determined as the highest value found in each specimen using the IHC Nuclear Image Analysis tool (Aperio Technologies, Vista, CA, USA) and was similarly measured and determined in cell-block sections of EUS-FNA specimens as described previously [15].
The prominent concern about EUS-FNA is whether WHO classification (grading) is possible with the biopsy specimens. We previously reported a study [15] about a comparison of grades of pNENs between resected and EUS-FNA specimens by Ki67 immunostaining. The concordance rate rose to 90 % when EUS-FNA samples contained more than 2000 neoplastic cells. In accordance with our previous study, we defined the cases whose neoplastic cells were insufficient for grading (less than 2000 cells) as tumors of ‘uncertain’ grade.
Analysis of KRAS mutation
Genetic analysis was performed on either the fresh specimens or formalin-fixed paraffin-embedded sections. After nucleic acids were extracted and amplified by polymerase chain reaction, gene mutations were analyzed by ABI PRISM 310 Genetic Analyzer (Applied Biosystems) or the Cycleave PCR assay (Takara Co., Ltd); the detail of which was described previously [16, 17].
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) software and P values <0.05 were considered statistically significant. Categorical variables are expressed as absolute (n) and relative (%) frequencies and were compared using the Chi squared test or Fisher’s exact test. Survival was analyzed using the Kaplan–Meier method with the log-rank test.
Results
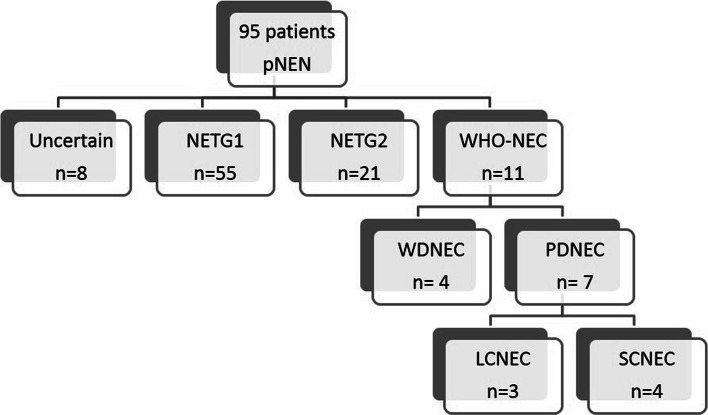
Ninety-five patients were diagnosed with pNEN at our hospital during the study period. As to grading of pNENs, the WHO classification 2010 suggests two parameters (mitotic count and Ki67 LI) to evaluate the proliferative activity of tumors. We performed grading of pNENs by measuring Ki67 LI and did not employ the mitotic count method, because our study consisted mostly of tumors diagnosed by FNA specimens, which were too small an amount to secure 50 microscopic fields necessary for the calculation of mitotic count. The pNENs were reclassified into uncertain for Ki67 LI (n = 8), NET-G1 (n = 55), NET-G2 (n = 21), and WHO-NEC (n = 11) in accordance with the WHO 2010 classification. The 11 cases of WHO-NEC were the subject of analysis in this study (Fig. 1).
Fig. 1.
Algorithm for patient selection from pNEN. NEN neuroendocrine neoplasm, NET neuroendocrine tumor, LCNEC large cell NEC, SCNEC small cell NEC, WDNEC well-differentiated neuroendocrine carcinoma, PDNEC poorly-differentiated neuroendocrine carcinoma
Basic demographic and clinical features of patients with WHO-NEC (Tables 1, 2)
Table 1.
Patient characteristics (n = 11)
| Gender | |
| Male/female | 6/5 |
| Age | |
| Median (range) | 59 years (28–74) |
| Symptom | |
| Yes (%) | 91 % (abdominal pain) |
| Site of pancreas tumor | |
| Head/body/tail | 2/5/4 |
| Tumor size | |
| Median (range) | 35 mm (20–55) |
| Metastasis | |
| Yes (%) | 72 % (liver metastasis) |
| Treatment | |
| Operation/chemotherapy/BSC | 2/8/1 |
Table 2.
Clinical, pathological features, treatment and response for chemotherapy of WHO-NEC patients
| Case | Age/sex | Location | Size (mm) | ENETS stage | Tissue sampling | Histology | Ki67 LI (%) | CGA | Synaptophysin | Rb | KRAS | Treatment | Response for platinum-based regimen |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30, M | Body | 45 | IIb | Biopsy and surgical resection | WDNEC | 40 | Positive | Positive | Positive | WT | Operation | ND |
| 2 | 59, F | Body | 30 | IIIb | Biopsy and surgical resection | PDNEC (small cell) | 80 | Positive | Positive | Positive | MT | Operation | ND |
| 3 | 49, F | Body | 35 | IV | Biopsy | PDNEC (large cell) | 85 | Positive | Positive | Negative | MT | CT (Gemcitabine) | ND |
| 4 | 68, F | Tail | 36 | IV | Biopsy | WDNEC | 48 | Positive | Positive | Positive | WT | CT (IP) | PD |
| 5 | 63, F | Body | 33 | IV | Biopsy | PDNEC (large cell) | 54 | Positive | Positive | Negative | MT | CT (IP) | PR |
| 6 | 61, M | Body | 45 | IV | Biopsy | PDNEC (large cell) | 90 | Positive | Positive | Negative | MT | CT (EP) | PR |
| 7 | 74, M | Head | 20 | IV | Biopsy | PDNEC (small cell) | 90 | Positive | Positive | Negative | WT | BSC | ND |
| 8 | 37, M | Head | 20 | IV | Biopsy | PDNEC (small cell) | 80 | Positive | Positive | Negative | MT | CT (EP) | PR |
| 9 | 50, F | Tail | 35 | IV | Biopsy | WDNEC | 45 | Negative | Positive | Positive | WT | CT (Everolimus) | ND |
| 10 | 55, M | Tail | 30 | IV | Biopsy | WDNEC | 53 | Positive | Positive | Positive | WT | CT (EP) | PD |
| 11 | 66, M | Tail | 70 | IV | Biopsy | PDNEC (small cell) | 95 | Positive | Positive | Negative | MT | CT (IP) | PR |
CGA chromogranin A, WT wild type, MT mutant, CT chemotherapy, IP cisplatin + irinotecan, EP cisplatin + etoposide, BSC best supportive care, ND not done, PD progressive disease, PR partial response
Ten (91 %) of 11 patients were symptomatic, mainly with abdominal pain. The median tumor size was 35 mm (range 20–55 mm). Tumors were located in the head, body, and tail of the pancreas in 2, 5, and 4 patients, respectively. Eight (72 %) patients had liver metastasis at the time of diagnosis, two were treated with surgery (ENETS stageIIb and IIIb) and six who received platinum-based chemotherapy (3 cases were cisplatin + irinotecan and 3 cases were cisplatin + etoposide) had a response rate of 67 %. In the remaining 2 patients, one patient received Gemcitabine (case 3) and another patient received Everolimus because we defined it as WDNEC (case 9). The overall median survival was 314 days (range 60–1202 days).
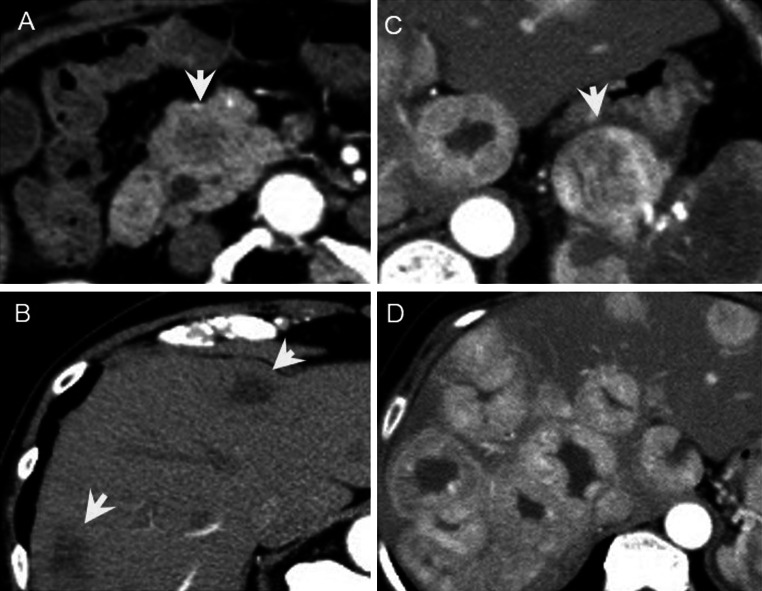
Imaging features of WHO-NEC on CE-MDCT (Fig. 2; Supplementary Table)
Fig. 2.
Computed tomography findings of respective pNECs. a, b Hypovascular lesions both primary pancreas head site and multiple liver lesions (SCNEC case). c, d Hypervascular lesions both primary pancreas head site and multiple liver lesions (WDNEC case)
Assessment by CE-MDCT revealed that 9 (82 %) of 11 WHO-NEC in the pancreas were hypovascular. Eight of these tumors had metastasized to the liver, where 7 (88 %) of them were also hypovascular, like the primary tumor (Fig. 2). Before biopsy confirmation, NEN were suspected in only two patients, and the imaging features in the remaining 9 (82 %), suggested pancreatic ductal adenocarcinoma (PDAC). The main pancreatic duct was dilated in 4 (57 %) of 7 patients with tumors located in the head and body of the pancreas.
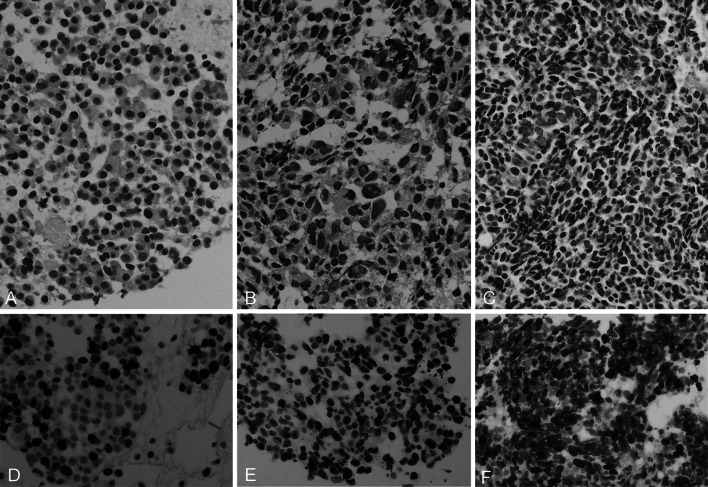
Pathological and molecular characteristics of WHO-NEC (Fig. 3, Supplementary Figure; Tables 2, 3)
Fig. 3.
Histologic features of NECs of the pancreas [H&E stain (a–c), and Ki67 (d–f), respectively]. The left column (a, d) is a case of WDNEC, the middle column (b, e) is of LCNEC, and the right column (c, f) is of SCNEC. Morphology of WDNECs shows a close similarity to that of NET-G1/G2, characterized by monomorphic growth of tumor cells with highly preserved endocrine cell features. Although LCNECs have features of endocrine cells as well, they are distinguished from WDNECs by increased nuclear atypia, cellular pleomorphism, and the frequent presence of tumor necrosis. SCNECs are composed of small cells with dense chromatin, scarce cytoplasm, and remarkable mitotic activity. These are reminiscent of small cell carcinomas of the lung
Table 3.
Pathological and molecular characteristics of WHO-NEC
| Ki67 labeling index | |
| Median (range) | 69.1 % (40–95 %) |
| Morphology | |
| WDNEC/PDNEC | 4/7 |
| Subtypes of PDNEC | |
| Large-cell type/small-cell type | 3/4 |
| Rb immunopositivity | 45 % (5/11) |
| KRAS mutation | 54 % (6/11) |
WDNEC well-differentiated NEC, PDNEC poorly differentiated NEC
A total of 11 WHO-NEC cases were submitted to the pathological and molecular analysis. No ductal carcinoma components were noted. All cases showed diffuse and strong immunoreactivity for neuroendocrine markers except 1 case, in which only synaptophysin was positive. In total, chromogranin A was expressed in 91 % and synaptophysin was expressed in 100 % of cases. The median Ki67 LI was 69.1 % (range 40–95 %). Nuclear expression of Rb protein was retained in 5 (45 %) tumors. KRAS mutations were detected in 6 (55 %) tumors. Seven (64 %) and 4 (36 %) of 11 tumors were categorized as PDNEC (4 SCNECs and 3 LCNECs) and WDNEC, respectively, according to their morphologic characteristics that we mentioned in the “Patients and methods” (Fig. 3, Supplementary Figure).
Clinicopathological comparison of well-differentiated and poorly differentiated NEC (Table 4)
Table 4.
Clinicopathological comparison of WDNEC and PDNEC
| WDNEC (n = 4) | PDNEC (n = 7) | |
|---|---|---|
| Vascularity in pancreas tumor | ||
| Yes (%) | 50 % (2/4) | 0 % (0/7) |
| Ki67 labeling index | ||
| Median (range) | 46.3 % (40–53 %) | 85 % (54–95 %) |
| Rb immunopositivity | 100 % (4/4) | 14 % (1/7) |
| KRAS mutation | 0 % (0/4) | 86 % (6/7) |
| Response rate of platinum-based regimen | 0 % (0/2) | 100 % (4/4) |
| Prognosis | ||
| Median | 227 days | 186 days |
WDNEC well-differentiated NEC, PDNEC poorly differentiated NEC
The clinicopathological comparison between the WDNEC and PDNEC groups revealed that they were clinically and molecularly different in several aspects as follows: hypervascularity in MDCT images, 50 % (2/4) vs. 0 % (0/7), P = 0.109; median Ki67 LI, 46 % (range 40–53 %) vs. 85 % (range 54–95 %), P = 0.001; nuclear expression of Rb, 100 % (4/4) vs. 14 % (1/7), P = 0.015; KRAS mutations, 0 % (0/4) vs. 86 % (6/7), P = 0.015; response rates to platinum-based chemotherapy, 0 % (0/2) vs. 100 % (4/4) P = 0.067; and median survival, 227 vs. 186 days, P = 0.227.
Discussion
When the WHO 2010 classification was applied to our patients with NENs of the pancreas, we found that 36 % of the high-grade category included tumors with well differentiated morphology. This critical finding has an impact on the treatment strategies, particularly the platinum-based chemotherapy which should be originally administered for only PDNEC.
Our findings suggested that WDNECs differ from PDNECs and are rather more closely related to NETs-G2 in terms of clinicopathological and molecular characteristics. Firstly, MDCT consistently showed hypervascularity in WDNEC, but not in PDNEC. Some reports indicated that tumor vascularity correlated with the proliferation index and/or WHO classification [18, 19]. Our findings indicated that only 18 % of WHO-NEC cases were suspected of pNEN according to imaging findings before EUS-FNA, with most being considered PDAC or pancreatic adeno-squamous carcinoma. That is, a significant proportion (82 %) of NECs could not be correctly diagnosed by imaging, especially the PDNEC type.
Histologically, WDNECs shared more morphological traits with NETs-G2 than PDNECs, allowing us to presume that WDNECs correspond to well-differentiated NETs with high proliferative activity. The Ki67 LI tended to be lower in WDNEC than in PDNEC. Notably, KRAS and Rb genes are promising molecular markers with which to distinguish these types of tumors. The result that KRAS mutations were not found in WDNECs supports the notion that this category lies in close proximity to NET-G2, as no pancreatic NETs-G1/G2 have been reported to possess KRAS mutations, whereas PDNECs have been shown to harbor KRAS mutations [10, 16, 20]. Loss of expression of Rb was found in 86 % of PDNEC cases, whereas all of the WDNEC cases retained its expression. Aberration of the Rb/p16 pathway has been reported to be frequently involved in PDNECs of the pancreas, gallbladder, and ampulla, but not in pancreatic well-differentiated NETs [10, 20–22]. Concerning pancreatic NEN, Yachida et al. [10] conducted immunohistochemical and genetic analyses of several oncogenes and tumor suppressor genes including KRAS and Rb, and revealed that the aberrations of both genes were common in PDNECs but none in NETs-G1/G2. Their conclusion that PDNECs were molecularly distinct from well-differentiated NETs is in keeping with our findings. Taken together, the difference between WDNEC and PDNEC appears to be clinically, histologically, and molecularly significant, and we consider that WDNECs are more likely to be in the category of well-differentiated NET rather than NEC, thus, favoring the designation, namely “NET-G3”.
Our study showed that both WDNEC and PDNEC patients harbored unfavorable outcome (median overall survival of 227 days and 186 days, respectively), which is in stark contrast to NET-G2 patients whose median overall survival is reportedly 162 months [1]. Although WDNEC and PDNEC shared aggressiveness clinically and pathologically, the efficacy of the treatment between them tended to be different; all WDNEC cases did not exhibit response to the platinum-based chemotherapy while all of the PDNEC cases did. The Nordic NEC study [9] found that WHO-NEC with Ki67 LI > 55 % responded to platinum-based chemotherapy, whereas those with Ki67 LI < 55 % did not. Although the Nordic NEC study mainly focused on the treatment and prognostic aspects, there was no detailed description of the pathologic characteristics of the cases. We suppose that some of their WHO-NEC included WDNEC as defined herein. Based on the results of the Nordic NEC study, the NCCN guidelines noted in footnotes that “intermediate Ki67 levels in the 20–50 % range may not respond well to platinum/etoposide as patients with small cell histology or extremely high Ki67 and so, a clinical judgment should be used”. When NEN is diagnosed as WHO-NEC, clinically the toxic platinum-based chemotherapy is usually administered as a first-line regimen. However, a recent case report showed a good response of high-grade NET to molecular targeted therapy with agents such as Everolimus [23]. In fact, one patient who was diagnosed with WDNEC and received Everolimus obtained partial response. The current WHO 2010 classification might be flawed in terms of the management of patients with NEC and the classification scheme for NECs should be revised as the clinical, pathological, and molecular characteristics of this high-grade NEN become more fully clarified.
In regard to IHC, chromogranin A was expressed in 91 % of WHO-NEC cases, and synaptophysin was expressed in 100 %. In a similar fashion, previous articles reported that chromogranin A was expressed in 81–94 %, and synaptophysin was expressed in 88–96 % [7–9]. Taken together, stainability of chromogranin A and synaptophysin is high not only in WDNEC but also in PDNEC.
In our institute, we perform EUS-FNA for the diagnosis of pancreatic tumors on a routine basis, and have been reported its usefulness so far [11, 14–16, 24]. The diagnostic accuracy of overall pancreatic tumors was 91.8 % (918/996) [14]. We previously detected KRAS mutations in 87 % (266/307) of EUS-FNA specimens from pancreatic masses in patients with PDAC [24] and none among 25 well-differentiated endocrine tumors [16]. Jiao et al. [20] also reported the absence of KRAS mutations in NET-G1/G2.
To the best of our knowledge, this is the first study which examined the clinicopathological characteristics of pNECs, with an emphasis on the difference between WDNEC and PDNEC. However, some limitations should be addressed. The retrospective design hindered precise analysis of all required data, imposed potential selection bias, and the patient cohort was small due to the natural rarity of pNECs that account for <1 % of all pancreatic carcinomas, and 2–7.5 % of all pNEN [2, 25]. Intratumoral heterogeneity is another important consideration. In our 11 cases of NEC, we did not note any adenocarcinoma component histologically nor immunohistochemically. Also, the result of the high frequency of Rb aberration in our series minimizes the possibility of a hidden presence of concomitant adenocarcinomas, as Rb aberration has been reported to be a rare event in PDACs (5–6 %) [26, 27]. Although the above observations do not fully rule out the possibility that some of the cases might contain an accompanying adenocarcinoma, this may be a relatively uncommon occurrence given the low frequency of an associated ductal adenocarcinoma in PDNECs reported by Basturk et al. [8] (6/44, 14 %). Finally, we address the feasibility of grading for pNENs diagnosed by FNA specimens, which constituted most of our series. Past studies of ours and of others claimed that grading by Ki67 LI can be applicable to FNA specimens by showing high concordance between the grade given by the FNA specimens and that by the corresponding resected specimens (concordance rate 78–90 %) [15, 28–31]. Indeed, downgrading or upgrading between G1 and G2 occurred in a small proportion of cases, but there was no tumor observed among the 5 studies that was graded as G3 by EUS-FNA and was downgraded to G2 by surgical resection. This observation, as well as the poor outcome of the current study, indicates that the admixture of ‘overestimated’ NETs-G2 in our cohort seemed unlikely to happen.
In conclusion, we identified a significant number of “WDNEC” cases among pNECs that were defined by the current WHO classification system. The clinicopathological and molecular analyses suggested that WDNEC is distinct from PDNEC. Though the number of cases we analyzed was limited, we believe that our scheme of subcategorizing pancreatic NEC showed promise. Further larger-scale studies are warranted to validate our stratification of WHO-NECs, which will facilitate a more personalized treatment of the patients with this rare malignant neoplasm.
Electronic supplementary material
Supplementary material 1 (TIFF 11224 kb) Supplementary Figure. Immunostaining for synaptophysin (A, B) and Rb (C, D) in WDNEC and PDNEC. (A) and (C) are the WDNEC case identical to that shown in Fig. 3a. (B) and (D) are the LCNEC case identical to that shown in Fig. 3b. The intermingled lymphocytes show positive nuclear staining of Rb, serving as a positive internal control (D, arrow)
Acknowledgments
This study was supported by a grant from the Pancreas Research Foundation of Japan and JSPS KAKENHI Grant Number 26461041.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- NEN
Neuroendocrine neoplasm
- WHO
World Health Organization
- NET
Neuroendocrine tumor
- NEC
Neuroendocrine carcinoma
- EUS-FNA
Endoscopic ultrasound-guided fine needle aspiration
- ENETS
European Neuroendocrine Tumor Society
- IHC
Immunohistochemistry
- PCR
Polymerase chain reaction
- SD
Standard deviation
- LCNEC
Large-cell NEC
- SCNEC
Small cell-NEC
- PDAC
Pancreatic ductal adenocarcinoma
Footnotes
S. Hijioka and W. Hosoda contributed equally to this work.
References
- 1.Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256–265. doi: 10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 2.Bosman F, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. Lyon, France: IARC Press; 2010.
- 3.Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG, Kvols LK, O’Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::AID-CNCR2820680202>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351–1355. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 7.Velayoudom-Cephise FL, Duvillard P, Foucan L, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endoc-Relat Cancer. 2013;20:649–657. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 8.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 10.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hijioka S, Matsuo K, Mizuno N, et al. Role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in diagnosing metastasis to the pancreas: a tertiary center experience. Pancreatol : Off J Int Assoc Pancreatol. 2011;11:390–398. doi: 10.1159/000330536. [DOI] [PubMed] [Google Scholar]
- 12.Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Archiv: Int J Pathol. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plockinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 14.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–981. doi: 10.1007/s00535-012-0695-8. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32–38. doi: 10.1055/s-0033-1359133. [DOI] [PubMed] [Google Scholar]
- 16.Hosoda W, Takagi T, Mizuno N, et al. Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Int. 2010;60:358–364. doi: 10.1111/j.1440-1827.2010.02527.x. [DOI] [PubMed] [Google Scholar]
- 17.Yatabe Y, Hida T, Horio Y, et al. A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn. 2006;8:335–341. doi: 10.2353/jmoldx.2006.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodallec M, Vilgrain V, Couvelard A, et al. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatol: Off J Int Assoc Pancreatol. 2006;6:77–85. doi: 10.1159/000090026. [DOI] [PubMed] [Google Scholar]
- 19.d’Assignies G, Couvelard A, Bahrami S, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors1. Radiology. 2009;250:407–416. doi: 10.1148/radiol.2501080291. [DOI] [PubMed] [Google Scholar]
- 20.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005;29:588–594. doi: 10.1097/01.pas.0000157974.05397.4f. [DOI] [PubMed] [Google Scholar]
- 22.Parwani AV, Geradts J, Caspers E, et al. Immunohistochemical and genetic analysis of non-small cell and small cell gallbladder carcinoma and their precursor lesions. Mod Pathol: Off J USA Can Acad Pathol Inc. 2003;16:299–308. doi: 10.1097/01.MP.0000062656.60581.AA. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca PJ, Uriol E, Galván JA, et al. Prolonged clinical benefit of Everolimus therapy in the management of high-grade pancreatic neuroendocrine carcinoma. Case Rep Oncol. 2013;6:441–449. doi: 10.1159/000354754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogura T, Yamao K, Sawaki A, et al. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769–774. doi: 10.1016/j.gie.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2014. doi:10.1007/s00535-014-0934-2. [DOI] [PubMed]
- 26.Barton CM, McKie AB, Hogg A, et al. Abnormalities of the RB1 and DCC tumor suppressor genes: uncommon in human pancreatic adenocarcinoma. Mol Carcinog. 1995;13:61–69. doi: 10.1002/mc.2940130202. [DOI] [PubMed] [Google Scholar]
- 27.Gerdes B, Ramaswamy A, Ziegler A, et al. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg. 2002;235:51–59. doi: 10.1097/00000658-200201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2013. doi:10.1111/cyt.12111. [DOI] [PubMed]
- 29.Piani C, Franchi GM, Cappelletti C, et al. Cytological Ki-67 in pancreatic endocrine tumours: an opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175–181. doi: 10.1677/ERC-07-0126. [DOI] [PubMed] [Google Scholar]
- 30.Larghi A, Capurso G, Carnuccio A, et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: a prospective study. Gastrointest Endosc. 2012;76:570–577. doi: 10.1016/j.gie.2012.04.477. [DOI] [PubMed] [Google Scholar]
- 31.Chatzipantelis P, Konstantinou P, Kaklamanos M, et al. The role of cytomorphology and proliferative activity in predicting biologic behavior of pancreatic neuroendocrine tumors: a study by endoscopic ultrasound-guided fine-needle aspiration cytology. Cancer. 2009;117:211–216. doi: 10.1002/cncy.20025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 11224 kb) Supplementary Figure. Immunostaining for synaptophysin (A, B) and Rb (C, D) in WDNEC and PDNEC. (A) and (C) are the WDNEC case identical to that shown in Fig. 3a. (B) and (D) are the LCNEC case identical to that shown in Fig. 3b. The intermingled lymphocytes show positive nuclear staining of Rb, serving as a positive internal control (D, arrow)