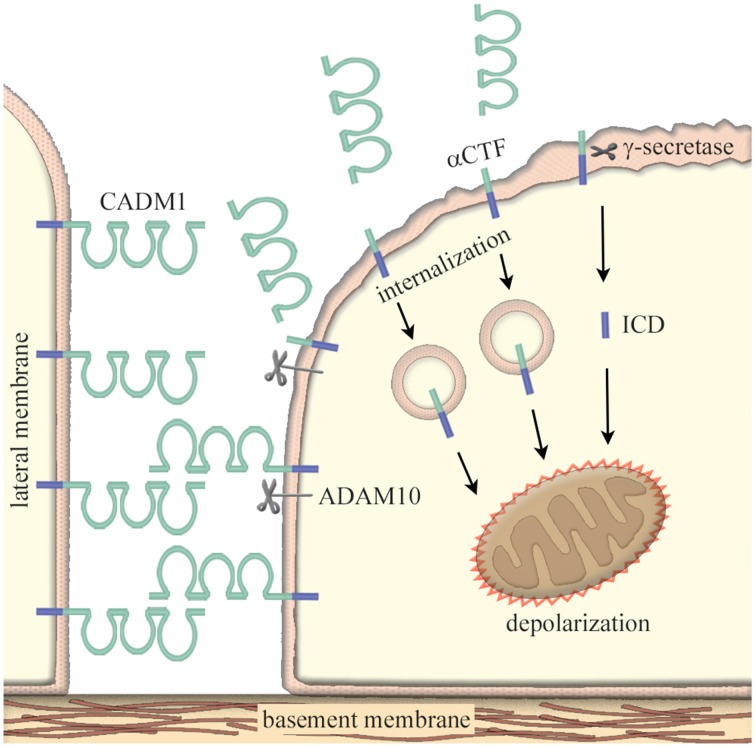
Figure 1.
Increased ectodomain shedding of CADM1 as a cause of epithelial cell apoptosis. In epithelia, CADM1 is located on the lateral cell membrane and mediates neighboring cell adhesion via trans-homophilic binding. Under pathological conditions, ectodomain shedding of CADM1 is induced through an imbalanced increase in protease activity such as neutrophil elastases, macrophage matrix metalloproteinases, and/or the inactivation of anti-protease α1-antitrypsin. CADM1 is shed at the extracellular domain by a disintegrin and metalloproteinase 10 (ADAM10) protease to produce a membrane-bound α C-terminal fragment (αCTF). Then, the truncated product αCTF enters into cytoplasm, accumulates in mitochondria, and depolarizes the mitochondrial outer membrane potential; this process results in mitochondrial apoptotic pathway. αCTF can be further cleaved within its transmembrane region by γ-secretase to produce free fragments of the intracellular domain (ICD). In similar to αCTF, ICD moves into mitochondria and induces apoptosis of lung epithelial cells. An increase in CADM1 shedding with an accompanying decrease in the full-length CADM1 induces the disruption of alveolar cell polarity and the cell apoptosis.