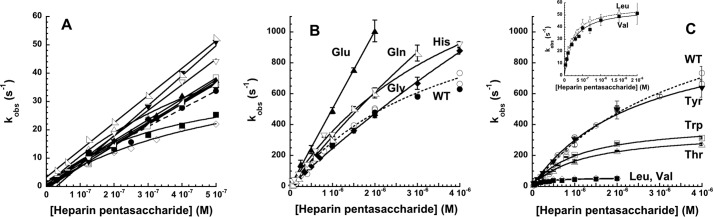
FIGURE 6.
Rapid kinetic analysis of heparin binding to select antithrombin P14 variants. Shown are plots of the heparin pentasaccharide concentration dependence of kobs for heparin pentasaccharide binding to antithrombin P14 variants in I 0.15 (A) and I 0.05 (B and C) sodium phosphate buffers, pH 7.4, 25 °C under pseudo-first order conditions. P14 variants in A are wild type (WT, ●), Thr (▵), Ala (▴), Met (○), Leu (♢), Val (■), Phe (□), Gly (♦), Pro (▷), His (▿), Tyr (▾), and Gln (◁); P14 variants in B and C are WT (○,●), His (▿), Gly (♦), Gln (◁), Glu (▴), Tyr (▾), Trp (□), Thr (▵), Leu (♢), and Val (■). Values of kobs were measured from heparin binding progress curves monitored by protein fluorescence changes for all except the Glu variant, which was monitored by TNS fluorescence changes (▴). Heparin binding to wild-type antithrombin was measured by both protein fluorescence (○) and TNS fluorescence changes (●). Solid lines in A are linear regression fits of data except for Thr, Leu, and Val variants that were fit by the hyperbolic Equation 16, whereas solid lines in B and C are fits of data by the hyperbolic Equation 16 except for the P14 Glu variant that was fit by linear regression. The dashed lines correspond to fits of wild type reactions monitored by protein fluorescence changes. The inset in C shows the P14 Leu and Val variant reactions on an expanded scale.