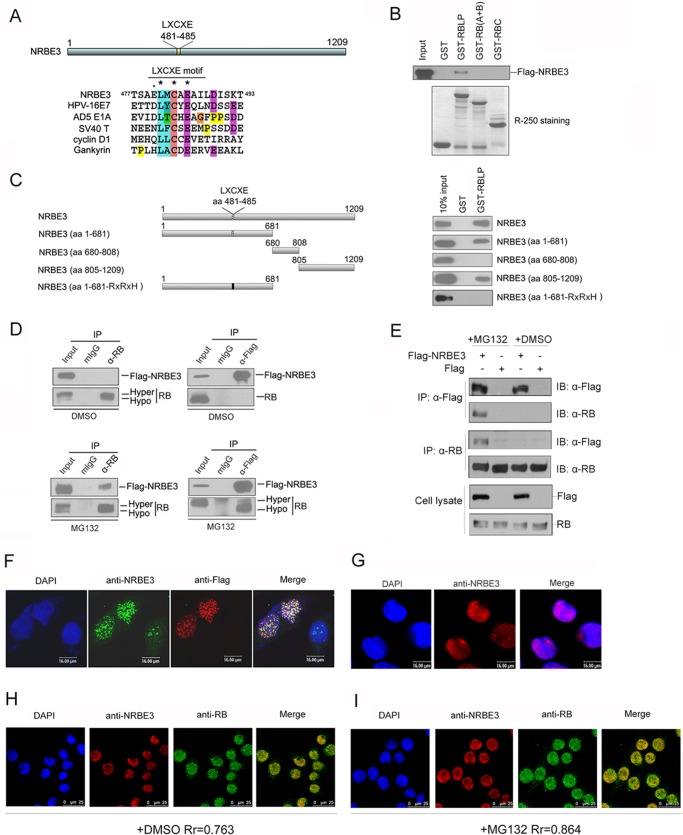
FIGURE 1.
NRBE3 interacted with RB in vitro and in vivo. A, schematic structure of NRBE3. The alignment of the LXCXE sequence in NRBE3 was plotted with that in HPV16 E7, AD5 E1A, SV40 large T antigen, cyclin D1, and gankyrin by ClustalW. B, GST pulldown was performed with E. coli-expressed GST or GST-RBLP, GST-RB (A+B), or GST-RBC fusion protein and in vitro transcribed/translated FLAG-NRBE3 protein. GST-RB-binding FLAG-NRBE3 proteins were evaluated by Western blotting with anti-FLAG antibody M2 (upper panel). Ten percent of the FLAG-NRBE3 protein was loaded as input control. GST or GST-RB deletion mutant proteins used in the GST pulldown experiment were stained with Coomassie Brilliant Blue R-250 as a loading control (lower panel). C, GST pulldown was performed with E. coli-expressed GST or GST-RBLP fusion proteins and in vitro transcribed/translated FLAG-NRBE3 and its deletion mutant proteins. GST-RBLP-binding FLAG-NRBE3 proteins were evaluated by Western blotting with anti-FLAG antibody M2 (right). Ten percent of FLAG-NRBE3 protein was loaded as input control. Schematic structures of FLAG-tagged NRBE3 deletion mutants are shown on the left. D, U2OS cells were transfected with FLAG-NRBE3, and immunoprecipitation was performed with anti-RB antibody (left) or anti-FLAG antibody (right). Immunoprecipitated RB and FLAG-NRBE3 proteins were evaluated by Western blotting with anti-RB or anti-FLAG antibody. Mouse IgG was used as a nonspecific antibody control. Five percent of cellular extracts for immunoprecipitation was loaded as input control. U2OS cells were transfected with FLAG-NRBE3, and cells were treated with the proteasome inhibitor MG132 for 4 h before harvest (lower). E, U2OS cells were transfected with FLAG-NRBE3 or FLAG, and immunoprecipitation were performed as described in D. F, HeLa cells were transfected with FLAG-NRBE3 expression plasmid. Cells were fixed 24 h post-transfection, and double immunofluorescence staining was performed with monoclonal anti-FLAG antibody M2 and polyclonal anti-NRBE3 antibody. Immunocomplexes were probed with TRITC-conjugated goat anti-mouse IgG and FITC-conjugated goat anti-rabbit IgG. Scale bars represent 16 μm. G, HeLa cells were fixed, and immunofluorescence staining was performed with polyclonal anti-NRBE3 antibody. TRITC-conjugated goat anti-rabbit IgG was used as the secondary antibody. Scale bars represent 16 μm. H, double immunofluorescence staining was performed with mouse monoclonal anti-RB and rabbit polyclonal anti-NRBE3 antibodies in HeLa cells. TRITC-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG were used as secondary antibodies. Scale bars represent 25 μm. I, HeLa cells were treated with MG132 before double immunofluorescence and immunofluorescence staining was performed as described in H. Nuclei were stained with DAPI. Scale bars represent 25 μm. The image was taken under confocal microscopy. Rr refers to the Pearson correlation coefficients. mIgG, mouse IgG; IP, immunoprecipitation; IB, immunoblot, DMSO, dimethyl sulfoxide; Hypo, hypophosphorylated; Hyper, hyperphosphorylated.