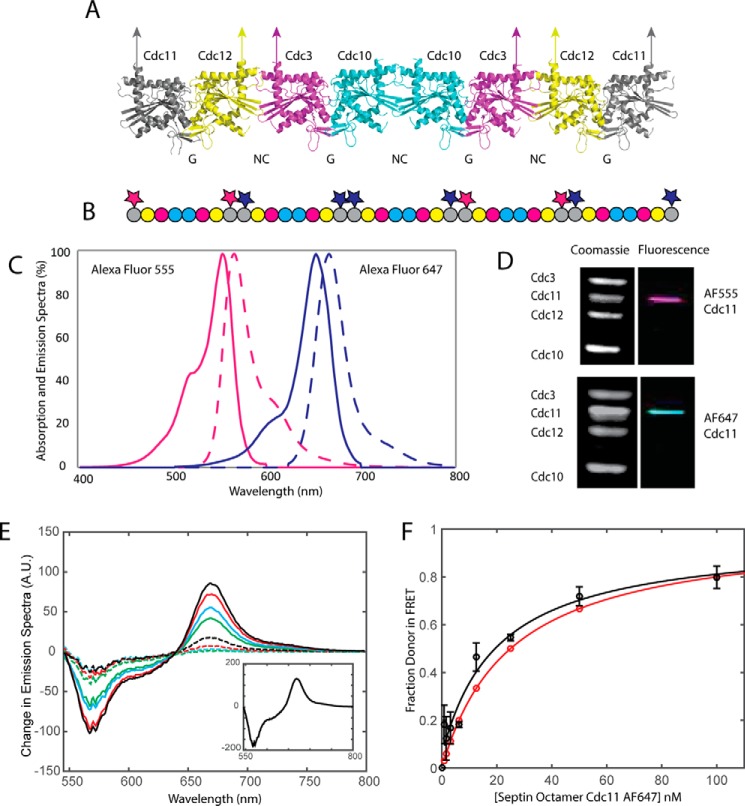
FIGURE 1.
Labeling terminal septin subunit (Cdc11) allows for establishment of a FRET system. A, schematic representation of subunits within the yeast septin octamer as predicted by Phyre II and aligned to the crystal structure of the human septin hexamer (2QAG) in PyMOL. B, schematic showing possible arrangements of Cdc11-labeled septin subunits. Donor- and acceptor-labeled septins are allowed to assemble stochastically. C, normalized excitation and emission spectra as measured of AF555 and AF647. D, Coomassie gels and typhoon scans of Cdc11 septin octamers labeled at the terminal septin with AF555 and AF647. E, FRET emission spectra with 25 nm Cdc11AF555-labeled octamers recorded at increasing concentrations of Cdc11AF647-labeled octamers: 0.78 nm (dashed green), 1.56 nm (dashed cyan), 3.13 nm (dashed red), 6.25 nm (dashed black), 12.5 nm (solid green), 25 nm (solid cyan), 50 nm (solid red), and 100 nm (solid black). The inset illustrates the result of the PCA. F, binding curve corresponding to fluorescence spectra in E. The black circles with S.E. error bars correspond to experimental data. The black line shows the fitted binding curve. The red curve is the predicted binding curve from stochastic assembly of the septin polymeric complex.