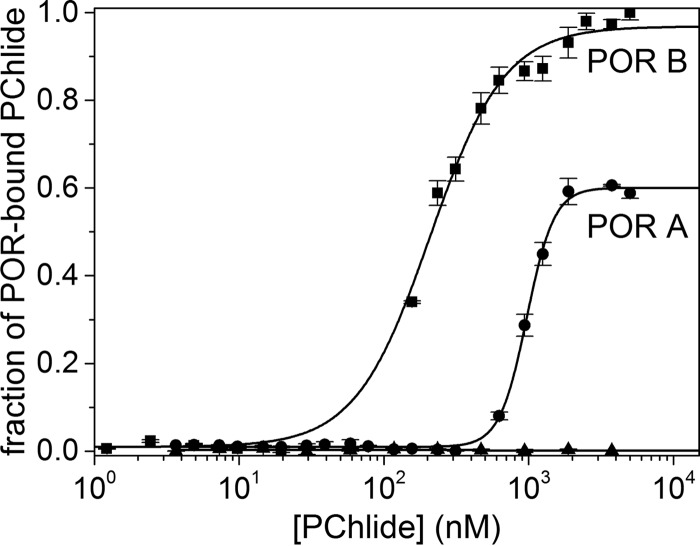
FIGURE 2.
Fraction of POR-bound PChlide in dependence on the concentration of added PChlide. The plots are shown for POR A (●) and POR B (■). The dissociation constant KD was estimated by fitting the binding curve with the quadratic solution for the fraction of the POR-NADPH complex that bound to PChlide, calculated from the law of mass action (Equation 6). The POR enzymes were labeled with the blue fluorescent dye NT-495. For the measurements POR-NADPH complexes (83 nm POR A and 166 nm POR B, as well as a 10-fold molar excess of NADPH) dissolved in measuring buffer (50 mm Tris-HCl, 0.3 m NaCl, 0.1% Triton X-100, 20% glycerol, 1 mm DTT, pH 7.6), were titrated with PChlide added in the concentration range from 1 nm to 5 μm. The experiments were repeated at least four times (n = 4). To exclude nonspecific binding a negative control containing only the labeled POR-NADPH complex in all capillaries was performed in parallel to the PChlide-bound POR-NADPH complex (▴). The corresponding data analysis indicates no binding at all.