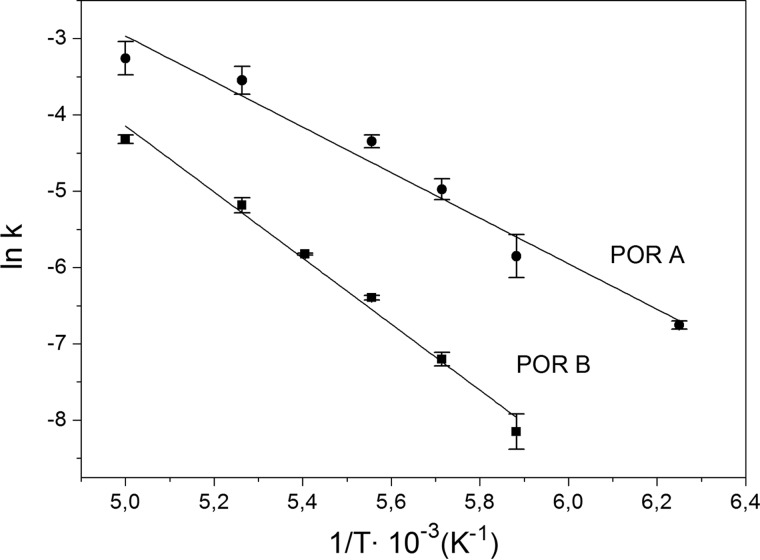
FIGURE 6.
Arrhenius plots displaying the logarithm of the rate constant (ln(k)) for the initial photoreaction of the ternary POR complex to the first photoproduct, A677, as a function of the inverse temperature (1/T). The plots are given for the reactions catalyzed by POR A (●) and POR B (■) in the temperature range from 160–200 K. From a fit of the data to the Arrhenius equation (Equation 8) the activation energies (ΔG#) are calculated to be 24.9 ± 2.1 kJ mol−1 for POR A and 35.9 ± 1.8 kJ mol−1 for POR B.