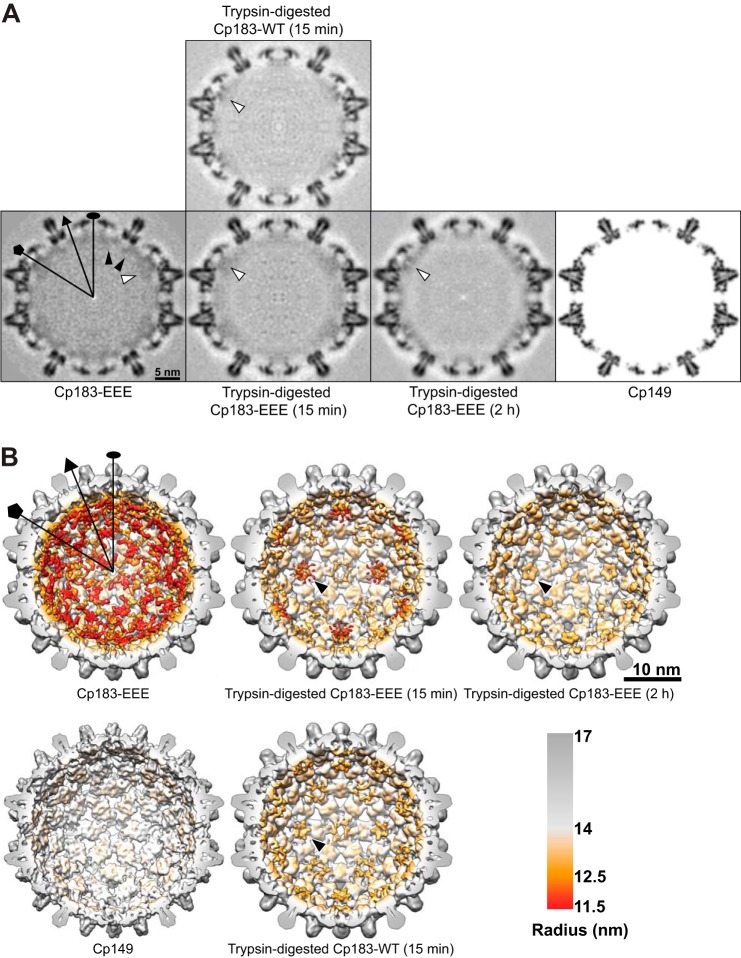
FIGURE 7.
Cryo-EM shows that CTD density underneath the five-fold symmetry axes is retained during trypsin digestion of Cp183-WT and Cp183-EEE capsids. Selected symmetry axes (icosahedral two-fold, three-fold, and five-fold) are marked as oval, triangle, and pentagon, respectively. A, central sections of the cryo-EM density maps of undigested and digested Cp183-EEE and Cp183-WT capsids are shown in gray scale. The central section of a Cp149 capsid, calculated from x-ray model (PDB entry 1QGT), to 9 Å resolution, is shown as a reference. Black arrowheads indicate quasi-six-fold associated CTD density, while white arrowheads indicate density underneath the five-fold symmetry axes. B, surface-shaded interior views of cryo-EM three-dimensional reconstructions of Cp183-EEE and Cp183-WT capsids were radially color-coded to highlight CTD density. The assembly domain is gray, and the CTD is orange-red. A model of a Cp149 capsid is shown as a reference. The front half of the particles were computationally removed to show the interior. The CTD density under the five-fold axis shows a petal-like organization (black arrows). CTD density under quasi-six-fold (two-fold) and quasi-three-fold axes disappeared with trypsin treatment.