Abstract
Oxidative stress may play a pathogenic role in chronic hepatitis C (CHC). The present study examined the oxidative status in plasma of patients with CHC who received pegylated interferon and ribavirin therapy. The following groups were included: (1) sustained virological response (28 patients), (2) null response (26 patients), (3) breakthrough (24 patients), (4) relapse (24 patients), (5) spontaneous cure (23 patients) and (6) twenty five normal subjects as a control group. Markers of oxidative stress including plasma malondialdehyde, nitric oxide, reduced glutathione, total antioxidant capacity and uric acid as well as serum ALT, AST, alkaline phosphatase, total bilirubin, albumin, prothrombin time were studied. The study indicated significant decline in reduced glutathione and total antioxidant capacity and markedly elevated levels of malondialdehyde and nitric oxide in all groups compared with the controls. Null response group had the highest levels of malondialdehyde and nitric oxide. Nitric oxide was significantly higher in those with null response compared with all other groups and with control subjects. Uric acid was significantly higher in spontaneous cure group compared with all other groups and with the controls. We concluded that CHC patients had increased oxidative stress. The oxidative status in plasma of these patients was not changed by antiviral therapy. The study also showed an important contribution of nitric oxide in null response patients. High serum uric acid did not interfere with the response and/or did not predict the response to antiviral therapy.
Keywords: chronic hepatitis C, interferon, oxidative stress
Introduction
Reactive oxygen species as well as reactive nitrogen species are products of normal cellular metabolism. The increased production of oxygen free radicals and nitric oxide occurs also during the respiratory burst of phagocytic cells which plays an essential role in bacterial killing and in regulating the process of acute inflammation (Horta et al., 2012[22]; Halliwell, 1982[18]). Under physiological conditions free radical generation is counter balanced by several mechanisms which aim to maintain the redox baklace in the cell. Cellular defenses against free radicals and reactive oxygen species include enzymatic and nonenzymatic mechanisms. The enzymatic defense consists mainly of catalase, reduced glutathione (GSH), glutathione peroxidase, and superoxide dismutase; the nonenzymatic antioxidant mechanisms include ascorbic acid, vitamin E and GSH (Halliwell and Gutteridge, 1984[20]). When these protective or counter mechanisms are overwhelmed by excessive generation of oxygen and nitrogen free radicals, oxidative and/or nitrosative stress then ensues. In these conditions the increased free radicals can damage cell structures including membrane lipids, proteins or DNA resulting in impaired cell function (Halliwell, 1996[17]; Kannan 2006[25]). Oxidative stress has been implicated in a number of human diseases such as liver disease, diabetes mellitus, heart failure, as well as in neurodegenerative disorders (Halliwell, 2009[19]).
Hepatitis C virus (HCV) infection is a leading cause of chronic hepatitis and cirrhosis wide-world. It is estimated that approximately 160-170 million people world-wide are affected with chronic hepatitis C (CHC) (Lavanchy, 2011[28]). Cirrhosis eventually develops in 30 - 40 % of individuals unless the virus is eradicated with antiviral therapy (Bruno and Facciotto, 2008[2]; Davis et al., 2010[4]). Chronic hepatitis C virus infection is also is associated with the highest hepatocellular carcinoma (HCC) incidence in persons with cirrhosis (Fattovich et al., 2004[13]). Current standard treatment for chronic hepatitis C includes pegylated interferon (IFN) alfa in combination with ribavirin (McHutchison et al., 2009[31], Guo et al., 2012[15]). The aim is to attain sustained virological response (SVR). The effectiveness of IFN-ribavirin in treating patients depends on HCV genotype. Patients with genotypes 2 and 3 experience the highest SVR rate, with rates in excess of 80 %. For genotype 1 HCV-infected patients, only 40-46 % of patients will achieve SVR (Shepherd et al., 2004[47]; Lee et al., 2012[29]). However, approximately 10 % of patients receiving INF-ribavirin therapy will stop treatment and 30 % of patients require dose reduction because of side effects (Lee et al., 2012[29]; Druyts et al., 2013[8]).
Recently, the role of oxidative stress in liver diseases and especially in patients with HCV infection has received considerable interest (Quarato et al., 2013[41]; Yen et al., 2012[55]; El-Kannishy et al., 2012[9]). Oxidative stress is a common pathogenetic mechanism contributing to initiation and progression of hepatic damage in a variety of liver disorders such as alcoholic liver disease, chronic viral hepatitis, autoimmune liver diseases and non-alcoholic steatohepatitis (Medina and Moreno-Otero, 2005[33]). During the progression of HCV infections, reactive oxygen species are generated, and these then induce significant DNA damage and the development of hepatocellular carcinoma (Tsukiyama-Kohara, 2012[52]). Hence, knowledge on the effect of IFN-ribavirin therapy on the oxidative status of patients with HCV infection is of marked clinical significance. Moreover, drugs with potential antioxidant activity are likely to be of benefit in the treatment with patients with chronic HCV infection. In this context, the potential benefit of silymarin (extracted from the seeds of Silybum marianum or milk thistle) in the treatment of liver diseases may also stem from scavenging of reactive oxygen species (Saller et al., 2001[44]). Silymarin supplemenation to antiviral therapy improved oxidative stress (Pár et al., 2009[35]) and supplementation with antioxidants such as vitamins E, C and zinc appear to improve the redox status in these patients (Farias et al., 2012[12]).
The aim of the present study was to assess the antioxidant status in chronic hepatitis C patients who received antiviral therapy with pegylated interferon and ribavirin.
Materials and Methods
Subjects
150 subjects were chosen in this study. 125 chronic hepatitis C (CHC) patients were included. They were recruited from the Hepatology clinics of the General Health Insurance Authority Polyclinics, in their first follow up visit, about two months after determining their response to their interferon therapy. Their combined interferon therapy was in the form of a fixed weekly dose of 160 μg of 20 kD linear pegylated interferon a-2a (Reiferon Retard) in combination with ribavirin in standard and adjusted doses. Their therapy was in accordance with the national protocols for the treatment of CHC approved by the Ministry of Health in Egypt (Taha et al., 2010[51]; Esmat and Abdel Fattah, 2009[11]). Subjects were fully informed and consented in advance about the nature of our study. Patients were divided into the following 4 groups according to their response to interferon therapy:
Sustained virological response (SVR) group (28 patients). SVR is defined as undetectable HCV-RNA in serum, even after 24 weeks withdrawal of standard combination therapy (Welker and Zeuzem, 2009[54]). In Egypt (genotype 4) the duration of treatment in this group was 48 weeks.
Null response group (26 patients). No response is defined as < 2 log (10) reduction in HCV RNA after 12 weeks treatment (Chayama et al., 2012[3]).
Breakthrough group (24 patients). The breakthrough response is defined when HCV RNA rebounds and becomes detectable before treatment is completed (Sherman et al., 2007[48]).
Relapse group (24 patients). virological relapse is defined if HCV RNA decreases and remains below the limit of detection during treatment but becomes detectable after cessation of treatment (Dieterich et al., 2009[7]).
Group 5 and 6 (who needed no treatment) were included as follows:
Spontaneous cure group (23 patients). Spontaneous HCV Resolution (Self-limiting HCV infection). The patient had at least 3 negative HCV-RNA PCR, 6 months apart with positive HCV-Ab done by ELISA on many occasions (Spada et al., 2004[49]).
The control group. This group included 25 age, sex and culture matched healthy volunteers with a normal medical history, physical examinations, blood biochemistry and negative for anti-HCV antibodies.
Exclusion criteria
Although many of the following criteria were excluded before enrollment in combined interferon therapy, we re-evaluated our subjects clinically and biochemically and excluded from this study those with a history of antioxidant use a month preceding the study, alcohol, smoking, other known cause of liver disease such as metabolic diseases, or another co-infections. Also subjects with other chronic diseases such as diabetes mellitus or coronary heart disease were excluded.
Biochemical studies
Liver function tests including alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and albumin were assayed using Olympus auto analyser AU400 (Olympus Diagnostica, Japan). Lipid peroxidation was assayed by measuring the level of malondialdehyde (MDA) in plasma using the method of Ruiz-Larrea et al (1994[43]). Reduced glutathione (GSH) was determined in brain tissue by Ellman's method (Ellman, 1959[10]). Nitric oxide in serum was measured as nitrite using Griess reagent, according to the method of Moshage et al. (1995[34]). Antioxidant capacity was measured by the reaction of antioxidants in the sample with a defined amount of exogenously provided hydrogen peroxide according to (Koracevic et al., 2001[26]) using commertially available kit (Biodiagnostics, Egypt). Uric acid concentration was measured by the direct enzymatic method, in which uric acid was oxidized by uricase coupled with peroxidase (Fossati et al., 1980[14]).
HCV antibodies determination
The presence of HCV antibodies was determined by third-generation enzyme linked immunosorbent assay (ELISA; CTK-Bioteck-USA).
Detection of HCV-RNA by Real Time PCR and HCV Genotyping
Viral RNA was extracted from patient's plasma using the QIAamp Viral RNA Kit (Qiagen Hilden, Germany, Cat no. 52904) according to the manufacturer's protocol. HCV RNA was detected by commercially available Toyobo RNA-direct real time PCR kit on SLAN Real Time PCR Detection System, LG Lifescience, Korea. The HCV genotype was defined by the reverse line probe assay (INNO-LIPA v.1.0, innogenetics, Ghent, Belgium) according to the manufacturer's instruction.
Statistical analysis of data
Data are expressed as mean ± S.E. Sta-tistical significance of the difference was analyzed using one way-ANOVA and post-hoc Duncan test for multiple group comparison. P values of < 0.05 were considered statistically significant.
Results
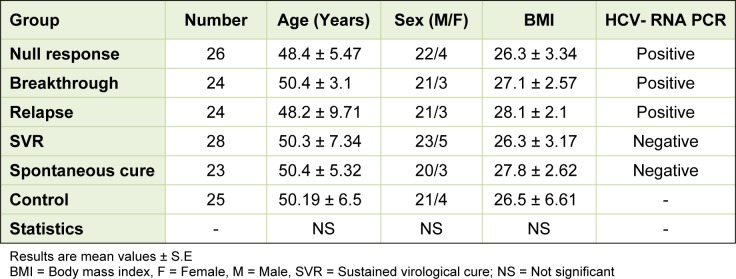
The demographic data of our subjects revealed no significant differences among the studied groups as regard age, sex and body mass index (Table 1(Tab. 1)). The number of the subjects and the HCV-RNA PCR status of each group were also shown in Table 1(Tab. 1).
Table 1. Characteristics of the selected groups.
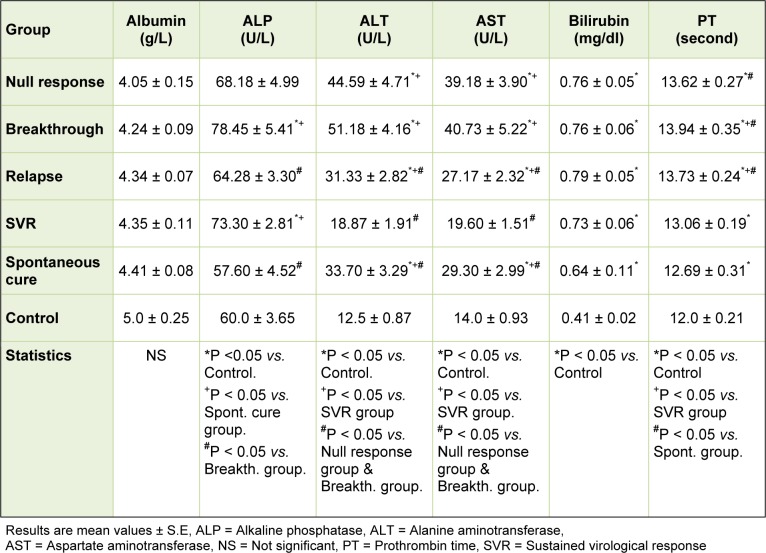
Concerning the studied parameters of liver function tests, there were no significant changes in the mean value of serum albumin levels among the different studied groups. The mean values of both ALT and AST exhibited significant increases in all patient groups when compared to that of the control group except for the SVR group which showed a non significant increase. Such mean values exhibited significant increases in all patient groups when compared to that of the SVR group. The null response and the breakthrough groups showed the highest significant mean values of ALT and AST. The mean values of serum bilirubin exhibited significant increases in all patient groups when compared to that of the control group. There were no significant changes in the mean levels of serum bilirubin among the patients groups. The mean values of serum alkaline phosphatase showed significant increases in the breakthrough and SVR groups and non significant increase in the null response and relapse groups when compared with the controls. Although within the accepted range, the mean values of prothrombin time were significantly increased in all patient groups when compared to that of the control group. Among the patients, the mean values of prothrombin time were significantly increased in all groups when compared to that of SVR group (Table 2(Tab. 2)).
Table 2. Serum levels of liver function parameters among different groups.
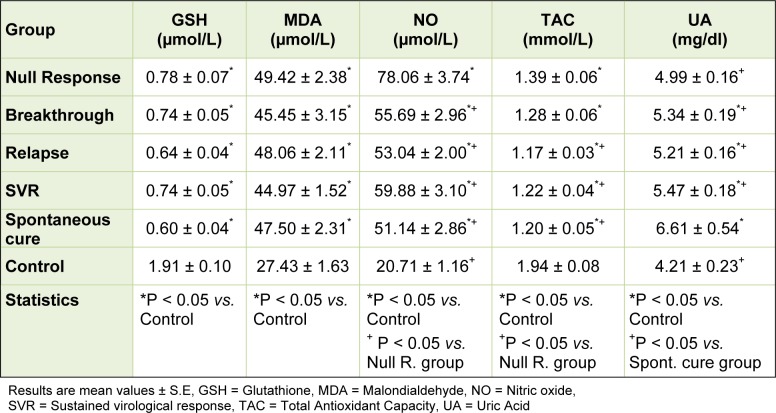
As regard the studied oxidative stress markers, there were significant decreases in the mean values of both reduced glutathione and total antioxidant capacity of all patients groups when compared with that of the control group. There were no significant differences in the mean reduced glutathione levels among the patients groups. The mean values of total antioxidant capacity were significantly lower in the relapse, SVR and spontaneous groups when compared with the null group. Both the mean values of malondialdehyde and nitric oxide showed significant increases in all patients groups when compared with that of the control group. There were no significant differences in the mean malondialdehyde levels among the patients groups. The mean levels of nitric oxide were significantly higher in the null response group compared with the means of all the other patients groups. The mean serum uric acid levels were significantly higher in all patients groups except the null response group when compared with that of the control group. Those who developed spontaneous cure showed the highest significant mean uric acid value. Other patients groups exhibited no significant change in the mean uric acid level among them (Table 3(Tab. 3)).
Table 3. Serum levels of oxidative stress markers among different groups.
Discussion
The present study indicated that our patients with chronic hepatitis C irrespective to their response to pegylated-interferon alpha2a plus ribavirin therapy have elevated levels of oxidative stress in their plasma.
The free-radical oxidation of polyunsaturated fatty acids in biological systems is known as lipid peroxidation. The detection and measurement of lipid peroxidation is the evidence most frequently cited to support the involvement of free-radical reactions in disease process. Aldehydes such as malondialdehyde resulting from lipid peroxidation can bind covalently to proteins, thereby altering their function which results in enzyme inhibition and alteration of the structure of cellular receptors and cellular damage (Gutteridge, 1995[16]). This study revealed increased malondialdehyde (MDA) in plasma of patients with chronic hepatitis C, suggesting increased free radical generation and lipid peroxidation. Nevertheless, there were no significant differences in the mean MDA level among different patient groups e.g., between those with null response or relapse and patients with sustained virological response. This finding might suggest that antiviral therapy had no effect on the level of oxidative stress in our patients.
Our results also demonstrated decreased endogenous antioxidant mechanisms in chronic hepatitis C. Reduced glutathione (GSH) was markedly decreased in the plasma of all the patients groups. Glutathione is a major antioxidant in tissues which participates nonenzymatically and enzymatically in supporting cellular redox balance and in protecting against oxidative damage by reactive oxygen species (Wang and Ballatori, 1998[53]). Normally, oxidized glutathione is efficiently reduced by glutathione reductase which maintains more than 98 % of intracellular gluathione in the reduced, thiol form (Spina and Cohen, 1998[50]).
Moreover, the study showed significant decrease in total antioxidant capacity (TAC) in all patients groups with chronic hepatitis C compared to the control group. Surprisingly, TAC was relatively increased in patients who had null response compared with relapse or spontaneous cure groups. Recently assays of TAC have been widely used in biomedicine with the aim of having an indication of oxidative status in body fluids or tissues. It has been suggested that low TAC could be indicative of oxidative stress. Conversely, increasing TAC through intervention has been taken as an indication of improved overall antioxidant status in the body. These assays, however, do not appear to reflect the sum of activities of different antioxidant defense mechanisms e.g., antioxidant enzymes (superoxide dismutases, glutathione peroxidases, and catalases) and glutathione which are the major antioxidant defense in cells, tissues, and body fluids or takes into account the contribution of small molecules e.g., urate, ascorbate, and tocopherol to antioxidant defense (Young, 2001[56]).
Nitric oxide (NO) is a short-living gaseous molecule and a biological mediator generated from L-arginine by NO synthase (NOS). Three isoforms of NOS have been identified; a constitutively expressed endothelial NOS (eNOS or type 3 NOS), neuronal NOS (nNOS or type 1 NOS) and an inducible NOS (iNOS or type 2 NOS). In the liver, constitutively generated nitric oxide is released continuously by the vascular endothelium. Being a vasodilator molecule, a key function of NO in the liver relates to the regulation of sinusoidal blood flow and vascular tone by exerting a basal dilator tone on the vascular smooth muscle cells (Rockey and Shah, 2004[42]). Nitric oxide thus helps to maintain the hepatic microcirculation and endothelial integrity. The role of nitric oxide in liver disease and especially in patients with hepatitis C virus infection has been emphasized by several authors. In diseased human livers, there is diminished activity of eNOS (Sarela et al., 1999[45]) and a significant increase in iNOS in the cirrhotic liver (McNaughton et al., 2002[32]). High concentrations of nitric oxide are generated by the inducible form of the enzyme nitric oxide synthase (iNOS) by activated macrophages during inflammatory states. This is a defense mechanism by which the body aims to eliminate infection. An excessively high level of nitric oxide that follows contributes to the inflammatory response and tissue damage and participates in the onset of several hepatopathies. However, in the course of liver regeneration, iNOS is expressed at moderate levels and contributes to inhibit apoptosis and to favor progression in the cell cycle until the organ size and function are restored (Martin-Sanz et al., 2002[30]). Lake-Bakaar et al. (2001[27]) found widespread expression of iNOS in hepatocytes in chronic HCV liver disease, irrespective of histological disease severity. The authors suggested that nitric oxide may play a role in the local control of chronic viral infections at tissue level, but this is not reflected in serum levels. Nitric oxide, however, is increased in the serum of patients with chronic HCV hepatitis (Pata et al., 2003[37]; Hokari et al., 2005[21]; Ibrahim et al., 2010[23]). In these subjects, nitric oxide levels appear to be related to the outcome of pegylated-IFN-α 2a plus ribavirin treatment (Hokari et al., 2005[21]; Ibrahim et al., 2010[23]). In chronic hepatitis C, responder patients in whom hepatitis C virus was eradicated had significantly higher nitric oxide than the non-responders, in whom the virus was not eradicated (Hokari et al., 2005[21]). This difference was seen as early as 2 weeks after the initiation of treatment (Hokari et al., 2005[21]). Conversely, suppression of nitric oxide might be a mechanism by which pegylated arginine deiminase reduces HCV viral titers and improves liver function in patients with chronic hepatitis C virus infection (Izzo et al., 2007[24]). Still other researchers observed that nitric oxide levels negatively correlated with fibrosis scores in chronic hepatitis patients. Nitric oxide which may be a suppressor molecule for fibrosing levels are inversely related to fibrotic process (Pata et al., 2003[37]). The present observations indicated increased nitric oxide in the serum of different groups including those with sustained virological response or spontaneous cure. The highest level of nitric oxide was, however, observed in patients who exhibited null response to antiviral therapy.
Recently there has been much interest in uric acid in relation to liver disease. In Chronic hepatitis C and in non alcoholic fatty liver disease patients, hyperuricaemia was independently associated with severity of steatosis (Petta et al., 2011[39], 2012[40]). Moreover, serum uric acid level > or =5.8 mg/dl was predictive of poor response to HCV treatment with pegylated-interferon and ribavirin (Pellicano et al., 2008[38]). In the present study, subjects with sustained response, breakthrough, relapse or even spontaneous cure had significantly higher levels of serum uric acid compared with controls. Uric acid was significantly higher in those who developed spontaneous cure compared with all other groups and with control subjects. Thus uric acid level in serum had no impact on the response to antiviral therapy in this study. Uric acid is recognized as a marker of oxidative stress whereas the production of the uric acid includes enzyme xanthine oxidase which is involved in producing of radical-oxygen species. On contrary to this, uric acid also acts is an "antioxidant", a free radical scavenger and a chelator of transitional metal ions which are converted to poorly reactive forms (Pasalic et al., 2012[36]). Uric acid is a natural antioxidant, accounting for up to 60 % of the free radical scavenging activity in human blood (Ames et al., 1981[1]). Uric acid forms stable co-ordination complexes with iron ions that inhibited Fe3+-catalysed ascorbate oxidation as well as lipid peroxidation (Sharma et al., 2012[46]). Uric acid also can scavenge peroxyl, hydroxyl and superoxide radicals and inhibit oxidative protein, DNA and lipids damage (Ames et al., 1981[1]; Davies et al., 1986[4]). In healthy subjects, systemic administration of uric acid which raised serum urate concentration from was associated with increased serum antioxidant capacity and reduced oxidative stress during acute physical exercise (Diaz et al., 2011[6]).
In summary, the present findings suggest an elevated levels of oxidative stress in the serum of patients with chronic hepatitis C infection. Both increased lipid peroxidation and decreased endogenous antioxidant mechanisms were observed. Oxidative stress was not changed by antiviral therapy. Supplementation with antioxidants might thus be advisable in these patients.
References
- 1.Ames B, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruno S, Facciotto C. The natural course of HCV infection and the need for treatment. Ann Hepatol. 2008;7:114–119. [PubMed] [Google Scholar]
- 3.Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–748. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 4.Davies KJ, Sevanian AS, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes: A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–21. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Brown MD. Oxidative Stress Response to Short Duration Bout of Submaximal Aerobic Exercise in Healthy Young Adults. Int J Ex Sci. 2011;4:247–256. [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich DT, Rizzetto M, Manns MP. Management of chronic hepatitis C patients who have relapsed or not responded to pegylated interferon alfa plus ribavirin. J Viral Hepat. 2009;16:833–843. doi: 10.1111/j.1365-2893.2009.01218.x. [DOI] [PubMed] [Google Scholar]
- 8.Druyts E, Thorlund K, Wu P, Kanters S, Yaya S, Cooper CL, et al. Efficacy and safety of pegylated interferon alfa-2a or alfa-2b plus ribavirin for the treatment of chronic hepatitis C in children and adolescents: a systematic review and meta-analysis. Clin Infect Dis. 2013;56:961–967. doi: 10.1093/cid/cis1031. [DOI] [PubMed] [Google Scholar]
- 9.El-Kannishy G, Arafa M, Abdelaal I, Elarman M, El-Mahdy R. Persistent oxidative stress in patients with chronic active hepatitis-C infection after antiviral therapy failure. Saudi J Gastroenterol. 2012;18:375–379. doi: 10.4103/1319-3767.103429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellman GL. Tissue sulfhydryl groups. Arch Biochem. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Esmat G, Abdel Fattah S. Evaluation of a novel pegylated interferon alpha-2a (Reiferon Retard) in Egyptian patients with chronic hepatitis C–genotype 4. Dig Liv Dis Suppl. 2009;3:17–9. [Google Scholar]
- 12.Farias MS, Budni P, Ribeiro CM, Parisotto EB, Santos CE, Dias JF, et al. Antioxidant supplementation attenuates oxidative stress in chronic hepatitis C patients. Gastroenterol Hepatol. 2012;35:386–394. doi: 10.1016/j.gastrohep.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Fossati P, Prencipe L, Berti G. Use of 3,5- dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980;26:227–231. [PubMed] [Google Scholar]
- 15.Guo X, Zhao Z, Xie J, Cai Q, Zhang X, Peng L, et al. Prediction of response to pegylated-interferon-α and ribavirin therapy in Chinese patients infected with different hepatitis C virus genotype. Virol J. 2012;9:123. doi: 10.1186/1743-422X-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutteridge J. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- 17.Halliwell B. Free radicals, protein and DNA: oxidative damage versus redox regulation. Biochem Soc Trans. 1996;24:1023–27. doi: 10.1042/bst0241023. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. Production of superoxide, hydrogen peroxide and hydroxyl radicals by phagocytic cells: a cause of chronic inflammatory disease? Cell Biol Int Rep. 1982;6:529–542. doi: 10.1016/0309-1651(82)90175-8. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B. The wanderings of a free radical. Free Radic Biol Med. 2009;46:531–42. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hokari A, Zeniya M, Esumi H, Ishikawa T, Kurasima Y, Toda G. Role of nitric oxide (NO) in interferon-alpha therapy for hepatitis C. J Infect. 2005;51:47–53. doi: 10.1016/j.jinf.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Horta MF, Mendes BP, Roma EH, Noronha FS, Macêdo JP, Oliveira LS, et al. Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J Parasitol Res. 2012 doi: 10.1155/2012/203818.. Available from: http://dx.doi.org/10.1155/2012/203818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim M, Gomaa W, Ibrahim Y, El Hadad H, Shatat M, Aleem AA, et al. Nitric oxide levels and sustained virological response to pegylated-interferon alpha2a plus ribavirin in chronic HCV genotype 4 hepatitis: A prospective study. J Gastrointest Liver Dis. 2010;19:387–392. [PubMed] [Google Scholar]
- 24.Izzo F, Montella M, Orlando AP, Nasti G, Beneduce G, Castello G, et al. Pegylated arginine deiminase lowers hepatitis C viral titers and inhibits nitric oxide synthesis. J Gastroenterol Hepatol. 2007;22:86–91. doi: 10.1111/j.1440-1746.2006.04463.x. [DOI] [PubMed] [Google Scholar]
- 25.Kannan S. Free radical theory of autoimmunity. Theor Biol Med Model. 2006;3:22. doi: 10.1186/1742-4682-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lake-Bakaar G, Sorbi D, Mazzoccoli V. Nitric oxide and chronic HCV and HIV infections. Dig Dis Sci. 2001;46:1072–1076. doi: 10.1023/a:1010770230422. [DOI] [PubMed] [Google Scholar]
- 28.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee LY, Tong CY, Wong T, Wilkinson M. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract. 2012;66:342–355. doi: 10.1111/j.1742-1241.2012.02895.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Sanz P, Hortelano S, Callejas NA, Goren N, Casado M, Zeini M, et al. Nitric oxide in liver inflammation and regeneration. Metab Brain Dis. 2002;17:325–334. doi: 10.1023/a:1021909902310. [DOI] [PubMed] [Google Scholar]
- 31.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. IDEAL Study Team. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 32.McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, et al. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci USA. 2002;99:17161–6. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina J, Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs. 2005;65:2445–2461. doi: 10.2165/00003495-200565170-00003. [DOI] [PubMed] [Google Scholar]
- 34.Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- 35.Pár A, Roth E, Miseta A, Hegedüs G, Pár G, Hunyady B, et al. Effects of supplementation with the antioxidant flavonoid, silymarin, in chronic hepatitis C patients treated with peginterferon + ribavirin. A placebo-controlled double blind study. Orv Hetil. 2009;150:73–9. doi: 10.1556/OH.2009.28517. [DOI] [PubMed] [Google Scholar]
- 36.Pasalic D, Marinkovic N, Feher-Turkovic L. Uric acid as one of the important factors in multifactorial disorders-facts and controversies. Biochem Med (Zagreb) 2012;22:63–75. doi: 10.11613/bm.2012.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pata C, Yazar A, Altintas E, Polat G, Aydin O, Tiftik N, et al. Serum levels of intercellular adhesion molecule-1 and nitric oxide in patients with chronic hepatitis related to hepatitis C virus: connection fibrosis. Hepatogastroenterology. 2003;50:794–797. [PubMed] [Google Scholar]
- 38.Pellicano R, Puglisi G, Ciancio A, Balzola F, Saracco G, Ciccone G, et al. Is serum uric acid a predictive factor of response to IFN-treatment in patients with chronic hepatitis C infection? J Med Virol. 2008;80:628–631. doi: 10.1002/jmv.21123. [DOI] [PubMed] [Google Scholar]
- 39.Petta S, Cammà C, Cabibi D, Di Marco V, Craxì A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;34:757–766. doi: 10.1111/j.1365-2036.2011.04788.x. [DOI] [PubMed] [Google Scholar]
- 40.Petta S, Macaluso FS, Cammà C, Marco VD, Cabibi D, Craxì A. Hyperuricaemia: another metabolic feature affecting the severity of chronic hepatitis because of HCV infection. Liver Int. 2012;32:1443–1450. doi: 10.1111/j.1478-3231.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- 41.Quarato G, Scrima R, Agriesti F, Moradpour D, Capitanio N, Piccoli C. Targeting mitochondria in the infection strategy of the hepatitis C virus. Int J Biochem Cell Biol. 2013;45:156–166. doi: 10.1016/j.biocel.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Rockey DC, Shah V. Nitric oxide biology and the liver: report of an aasld research workshop. Hepatology. 2004;39:250–257. doi: 10.1002/hep.20034. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–8. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 44.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–63. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 45.Sarela AI, Mihaimeed FM, Batten JJ, Davidson BR, Mathie RT. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut. 1999;44:749–53. doi: 10.1136/gut.44.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Botany. 2012;2012:1–26. [Google Scholar]
- 47.Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8:iii–iv,1. doi: 10.3310/hta8390. [DOI] [PubMed] [Google Scholar]
- 48.Sherman KE, Fleischer R, Laessig K, Murray J, Tauber W, Birnkrant D. Development of novel agents for the treatment of chronic hepatitis C infection: summary of the FDA Antiviral Products Advisory Committee recommendations. Hepatology. 2007;46:. 2014–. 2020. doi: 10.1002/hep.21985. [DOI] [PubMed] [Google Scholar]
- 49.Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia A, et al. Multispecific T cell response and negative HCV-RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004;53:1673–1681. doi: 10.1136/gut.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spina MB, Cohen G. Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci USA. 1989;86:1398–400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taha AA, El-Ray A, El-Ghannam M, Mounir B. Efficacy and safety of a novel pegylated interferon alpha-2a in Egyptian patients with genotype 4 chronic hepatitis C. Can J Gastroenterol. 2010;24:597–602. doi: 10.1155/2010/717845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsukiyama-Kohara K. Role of oxidative stress in hepatocarcinogenesis induced by hepatitis C virus. Int J Mol Sci. 2012;13:15271–15278. doi: 10.3390/ijms131115271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- 54.Welker MW, Zeuzem S. Occult hepatitis C: how convincing are the current data? Hepatology. 2009;49:665–75. doi: 10.1002/hep.22706. [DOI] [PubMed] [Google Scholar]
- 55.Yen HH, Shih KL, Lin TT, Su WW, Soon MS, Liu CS. Decreased mitochondrial deoxyribonucleic acid and increased oxidative damage in chronic hepatitis C.World J Gastroenterol. 2012;18:5084–5089. doi: 10.3748/wjg.v18.i36.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young IS. Measurement of total antioxidant capacity. J Clin Pathol. 2001;54:339. doi: 10.1136/jcp.54.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]