Abstract
A 68-year-old man diagnosed with cT3N2 adenocarcinoma of the rectum presented with a synchronous solitary liver metastasis on CT scan. Neoadjuvant chemoradiotherapy was started to downstage the primary tumour. Resection of the rectal tumour followed 3 months after the last radiotherapy session and primary resection of the isolated liver lesion was performed in the intervening period. Histopathological assessment of the liver lesion, however, showed no malignancy, but did reveal a necrotic infection due to Enterobius vermicularis. This parasite is frequently found in the intestines, but only rarely infects the liver. The patient was subsequently treated with the anthelmintic drug mebendazole 100 mg once a week for 2 weeks. Histopathological assessment of the rectal specimen showed complete regression after neoadjuvant chemoradiotherapy without evidence of remaining E. vermicularis, suggesting pinworm eradication. The patient recovered promptly after both surgical procedures.
Background
Colorectal liver metastases develop in approximately 50% of patients with colorectal cancer.1 Up to 25% of these patients present with synchronous liver metastases.2 Enhanced CT scan is the most commonly used imaging modality to assess liver metastases. The sensitivity and specificity to detect liver metastases by enhanced CT scan has been reported to be 69–71% and 86–91%, respectively.3 Owing to the risk of needle track deposits in approximately 19% of patients, which leads to a significantly worse oncological outcome, confirmation of the diagnosis of potentially resectable colorectal liver metastases by needle biopsy is generally avoided.4 However, this management option strategy bears the (limited) risk of a non-malignant liver lesion being unnecessarily treated as a colorectal metastasis due to its appearance on CT scan. In this case report, histopathological assessment of a liver resection performed in a patient with a preoperative CT diagnosis of a synchronous isolated colorectal liver metastasis, eventually revealed a benign lesion with Enterobius vermicularis infection of the liver.
With this case, we would like to emphasise that preoperative radiological diagnosis of liver lesions in patients with colorectal cancer is not accurate in all cases. According to this undesirable finding, we discuss the benefits and drawbacks of preoperative histological confirmation by liver biopsy compared with unnecessary liver resection.
Case presentation
A 68-year-old man presented to our hospital due to rectal bleeding and change in bowel habits during the past 5 months. The patient's medical history only included appendectomy at the age of 8 years.
Investigations
Colonoscopy revealed a suspicious lesion 15 cm from the anal verge, which was confirmed as adenocarcinoma after histological biopsy. A pelvic MRI showed a rectal location of the tumour, and it was classified as cT3N2, according to the TNM classification. Staging CT scan of the chest and abdomen demonstrated an isolated liver metastasis in segment 7 without signs of other distant metastases (figure 1). After discussion during the multidisciplinary team meeting, neoadjuvant chemoradiotherapy was started to downstage the primary tumour. After the last radiotherapy session, CT scan of the chest and abdomen was repeated, showing an unchanged isolated metastasis in segment 7 of the liver without any evidence of additional distant metastases.
Figure 1.
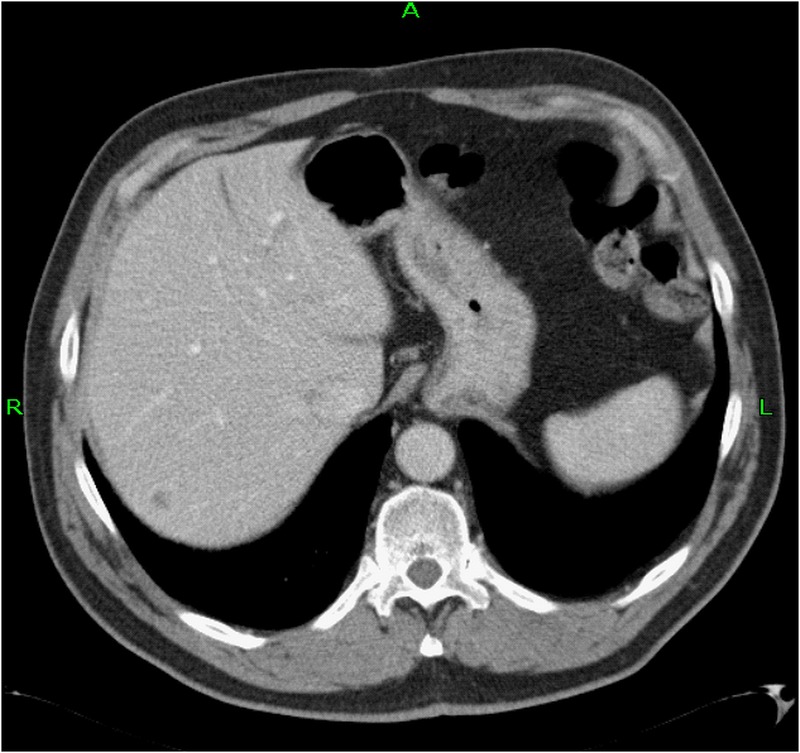
Staging CT scan of the chest and abdomen demonstrated an isolated metastasis in segment 7 of the liver (there were no signs of other distant metastases).
Treatment
During the multidisciplinary team meeting, it was decided to plan for resection of the rectal tumour 3 months after the last radiotherapy session. Primary resection of the isolated liver metastasis was performed in the intervening period.
During the liver resection, intraoperative ultrasonography revealed no additional liver metastases. Non-anatomical resection of liver segment 7 was subsequently performed. Postoperative recovery was uncomplicated and the patient was discharged 3 days postoperatively.
Outcome and follow-up
The pathologist's macroscopic assessment of liver segment 7 showed a relatively well-demarcated and soft lesion measuring 5 mm (figure 2). Microscopic evaluation of this lesion displayed a necrotic and granulomatous mass surrounded by a fibrotic pseudocapsule with a dense infiltrate of lymphocytes, plasma cells, macrophages and conspicuous eosinophilic granulocytes. The surrounding liver parenchyma showed no anomaly apart from a very mild infiltrate surrounding the portal triads (figure 3). Within the necrotic mass, one parasite, with a diameter of approximately 0.3 mm and narrow lateral alae characteristic of E. vermicularis (figure 4), was found. It was a gravid female pinworm containing multiple eggs. No metastasis of rectal carcinoma was found in the resection specimen. The patient was subsequently treated with the anthelmintic drug mebendazole 100 mg once a week for 2 weeks, to eliminate any possibly remaining E. vermicularis.
Figure 2.
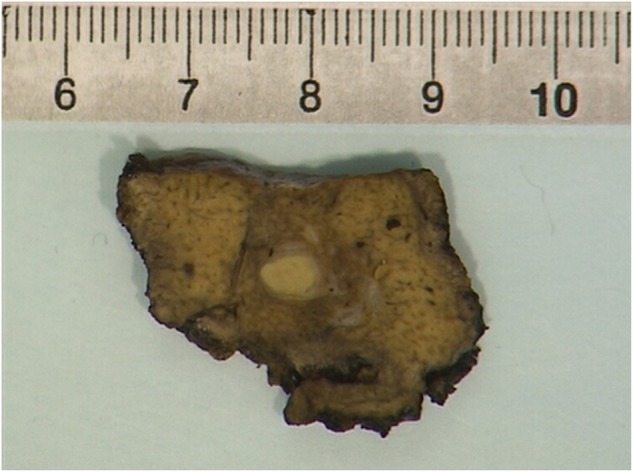
Partial resection of liver segment 7 displays a soft, round lesion, suspected of colorectal carcinoma metastasis.
Figure 3.
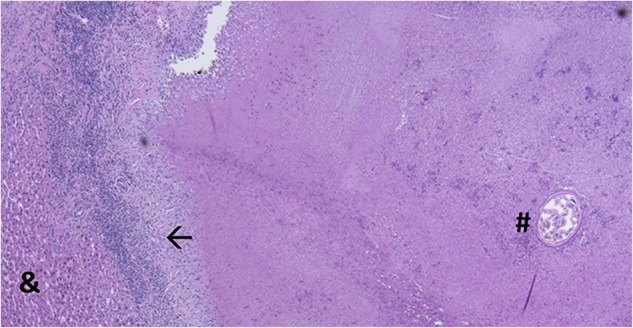
Microscopic analysis of the lesion demonstrates a necrotic mass surrounded by a fibrotic pseudocapsule with an inflammatory infiltrate (←). One pinworm (#) was identified in the necrotic mass. There was no colorectal metastasis found in the specimen. Pre-existing liver parenchyma can be seen on the left (&).
Figure 4.
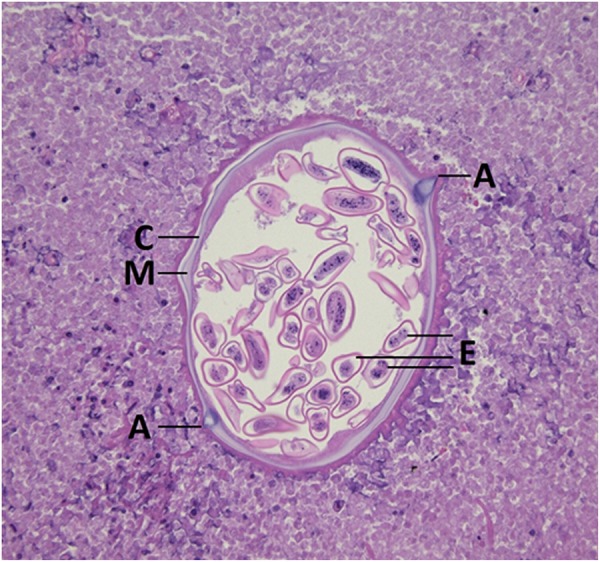
Gravid female Enterobius vermicularis with cuticle (C), contractile muscle (M), characteristic alae (A) and distended uterus with eggs (E).
MRI of the pelvis was repeated prior to resection of the primary tumour and showed a significant volume reduction of the rectal tumour. Laparoscopic low anterior resection with a defunctioning loop ileostomy was performed and the patient recovered with no postoperative complications. Histopathological assessment of the rectal specimen showed complete regression after neoadjuvant chemoradiotherapy (ypT0N0), without evidence of remaining E. vermicularis, suggesting pinworm eradication. The defunctioning ileostomy was reversed 2 months after low anterior resection. The patient recovered promptly after this final surgery.
Discussion
Surgery is currently the standard of care in patients with resectable colorectal liver metastasis without evidence for other distant metastases, except a limited number of pulmonary metastases.5 In the current case, the patient presented with an isolated synchronous colorectal liver metastasis as suspected by enhanced CT scan of the liver. Unexpectedly, however, histopathological assessment of the liver specimen showed a necrotic mass with a granulomatous inflammation due to infection with E. vermicularis. This nematode (roundworm) is the most common intestinal parasite (helminth) in Western Europe and the USA. Humans are the only known host. Prevalence is highest among the paediatric population. Spread of the parasite occurs via the faeco-oral route and so it is acquired by ingestion of the helminth ova (eggs) through contaminated hands and food. Usually, enterobiasis is asymptomatic or causes only mild symptoms. The most common symptom is nocturnal anal pruritus due to migration to the anus and deposition of ova perianally by the female gravid helminth.6
In children, infection of the small intestine, appendix and caecum by this parasite is common. However, it is an extremely rare finding in the liver.7 Only five cases have been previously reported. It is unclear whether this rare localisation of E. vermicularis is caused by direct migration or haematogenous spread, or if this condition is more common in patients with colorectal malignancy.8–13 Migration of E. vermicularis to the liver is only possible in case the intestinal mucosa is affected, since the mouth parts of E. vermicularis are considered unable to penetrate through healthy tissue.9 In the present case of female pinworms located in the liver of a man with a history of a colorectal tumour, it is most likely that the pinworms entered the portal circulation through a mesenteric vein that was damaged by tumour invasion.11
Nevertheless, preoperative differentiation between a malignant tumour and necrotising infection due to this parasite is, of course, desirable in order to prevent unnecessary surgery. However, routine ultrasound and CT scan were unable to differentiate between these two pathologies.13 During revision of the CT scan in the present case after the benign histological outcome of the presumed liver metastasis, the liver lesion was still highly suspicious for colorectal liver metastasis in this patient with rectal cancer, and did not indicate a benign lesion such as E. vermicularis. Other diagnostic imaging modalities, including MRI and positron emission tomography, would not be of significant value in differentiating between colorectal liver metastasis and a benign infection with E. vermicularis. Additionally, carcinoembryonic antigen (CEA) is used to monitor treatment and to identify recurrences after surgical resection of colorectal cancer and liver metastasis. In patients with suspected metachronous colorectal liver metastasis on imaging, without other signs of recurrence, measuring CEA can be useful to differentiate between metastasis and a benign liver lesion, by ruling out a benign lesion in the case of elevated CEA. However, in the case of synchronous liver metastasis, as in the current case, measuring CEA cannot differentiate between them, as CEA may solely be elevated by the primary tumour.14
The finding in the current case raises the question as to whether preoperative histological biopsy of a liver lesion is necessary in patients with suspected colorectal liver metastases, despite the risk of needle track deposits, or whether a liver resection for a benign lesion is acceptable in a small subset of patients. The chance of a benign histological outcome of the resection material after hepatic resection of a presumed colorectal liver metastasis is only 2.5%,15 while the risk of needle track deposits is common, occurring in approximately 19% of patients.4 Additionally, biopsy of colorectal liver metastasis leads to a significantly worse oncological outcome.4 Despite the morbidity of 13% after partial hepatectomy in patients with colorectal liver metastasis,16 we propose, based on our clinical experience and evidence in the current literature, that the small risk of an unwarranted resection is acceptable and that biopsy therefore should be avoided.
In conclusion, based on this case report, we would like to emphasise that the preoperative radiological diagnosis of liver lesions in patients with colorectal cancer is not accurate in all cases. However, the risk of tumour needle track deposits outweighs the benefits of preoperatively diagnosing a benign lesion in CT scan-suspected colorectal liver metastasis, due to the extremely low prevalence of this finding.
Learning points.
Preoperative radiological diagnosis of liver metastases in patients with colorectal cancer is not accurate in all cases.
Preoperative differentiation between a colorectal liver metastasis and a benign lesion by histopathological biopsy is not desirable in order to prevent unnecessary surgery, due to the relatively high risk of needle track tumour deposits.
Necrotising infection of the liver due to Enterobius vermicularis, as presented in this case, is an extremely rare finding in adult patients.
The prevalence of a benign lesion found in a liver specimen after surgery for suspected colorectal liver metastasis is very low.
Acknowledgments
The authors would like to thank Dr Steven Thijsen (Staff Clinical Microbiologist, Diakonessenhuis Utrecht, Utrecht), Dr Joost van Gorp (Staff Pathologist, Diakonessenhuis Utrecht, Utrecht) and Dr Anya Milne (Staff Pathologist, Diakonessenhuis Utrecht, Utrecht), for the time and effort they placed in reviewing the article.
Footnotes
Contributors: All the authors made individual contributions to the writing of the manuscript. EJBF and NS were the treating physicians. All the authors reviewed and edited the manuscript, and approved the final version.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 1989;210:127–38. 10.1097/00000658-198908000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C et al. . Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254–9. 10.1097/01.sla.0000217629.94941.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J. Imaging of hepatic metastases. Cancer Control 2006;13:6–12. [DOI] [PubMed] [Google Scholar]
- 4.Jones OM, Rees M, John TG et al. . Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br J Surg 2005;92:1165–8. 10.1002/bjs.4888 [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP et al. . Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–80. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 6.Grencis RK, Cooper ES. Enterobius, Trichuris, Capillaria, and hookworm including Ancylostoma caninum. Gastroenterol Clin North Am 1996;25:579–97. 10.1016/S0889-8553(05)70264-8 [DOI] [PubMed] [Google Scholar]
- 7.Meyers W, Neafie R, Marty A et al. . Pathology of infectious disease, volume I Helminthiases. AFIP, 2000. ISBN: 1881041654 [Google Scholar]
- 8.Little MD, Cuello CJ, D'Alessandro A. Granuloma of the liver due to Enterobius vermicularis. Report of a case. Am J Trop Med Hyg 1973;22:567–9. [DOI] [PubMed] [Google Scholar]
- 9.Daly JJ, Baker GF. Pinworm granuloma of the liver. Am J Trop Med Hyg 1984;33:62–4. [DOI] [PubMed] [Google Scholar]
- 10.Mondou EN, Gnepp DR. Hepatic granuloma resulting from Enterobius vermicularis. Am J Clin Pathol 1989;91:97–100. [DOI] [PubMed] [Google Scholar]
- 11.Arkoulis N, Zerbinis H, Simatos G et al. . Enterobius vermicularis (pinworm) infection of the liver mimicking malignancy: presentation of a new case and review of current literature. Int J Surg Case Rep 2012;3:6–9. 10.1016/j.ijscr.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts KJ, Hubscher S, Mangat K et al. . Pinworm infection masquerading as colorectal liver metastasis. Ann R Coll Surg Engl 2012;94:e195–7. 10.1308/003588412X13373405384891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou YM, Li B, Xu F et al. . Clinical features of solitary necrotic nodule of the liver. Hepatobiliary Pancreat Dis Int 2008;7:485–9. [PubMed] [Google Scholar]
- 14.Verberne CJ, Wiggers T, Vermeulen KM et al. . Detection of recurrences during follow-up after liver surgery for colorectal metastases: both carcinoembryonic antigen (CEA) and imaging are important. Ann Surg Oncol 2013;20:457–63. 10.1245/s10434-012-2629-3 [DOI] [PubMed] [Google Scholar]
- 15.Clayton RA, Clarke DL, Currie EJ et al. . Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon 2003;1:32–8. 10.1016/S1479-666X(03)80006-9 [DOI] [PubMed] [Google Scholar]
- 16.Kneuertz PJ, Pitt HA, Bilimoria KY et al. . Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg 2012;16:1727–35. 10.1007/s11605-012-1938-y [DOI] [PubMed] [Google Scholar]


