Abstract
Castleman disease (CD) is a rare and heterogeneous disorder characterized by lymphadenopathy that may occur in a single lymph node (unicentric) or multiple lymph nodes (multicentric), the latter typically occurring secondary to excessive proinflammatory hypercytokinemia. While a cohort of multicentric Castleman disease (MCD) cases are caused by Human Herpes Virus-8 (HHV-8), the etiology of HHV-8 negative, idiopathic MCD (iMCD), remains unknown. Breakthroughs in “omics” technologies that have facilitated the development of precision medicine hold promise for elucidating disease pathogenesis and identifying novel therapies for iMCD. However, in order to leverage precision medicine approaches in rare diseases like CD, stakeholders need to overcome several challenges. To address these challenges, the Castleman Disease Collaborative Network (CDCN) was founded in 2012. In the past 3 years, the CDCN has worked to transform the understanding of the pathogenesis of CD, funded and initiated genomics and proteomics research, and united international experts in a collaborative effort to accelerate progress for CD patients. The CDCN’s collaborative structure leverages the tools of precision medicine and serves as a model for both scientific discovery and advancing patient care.
Keywords: Castleman disease, lymphoproliferative disorder, precision medicine, genomics, Interleukin-6, orphan disease, rare disease
Introduction
Precision medicine describes the use of an individual’s “omics” data (genomics, transcriptomics, proteomics, and metabolomics) to guide decisions in clinical practice. In this approach, the diagnosis, management, and prevention of disease are tailored or “personalized” to a specific patient’s profile [1]. This approach is particularly important for rare diseases, which affect 8 to 10 percent of the U.S. population [2]. It is estimated that approximately 80 percent of rare diseases have a genetic origin [3], and the rest of rare diseases are in a position to benefit from transcriptomics and proteomics technologies that have been enabled by the “omics” revolution.
“Omics” data can be used to elucidate the etiology of a rare disease as well as to personalize management for a particular rare disease patient based on that individual’s features. An excellent example of a rare disease in which the precision medicine approach has already enabled breakthroughs for the etiology of the disease and for personalizing individual therapies is cystic fibrosis (CF). Sequencing was used to identify the causative CFTR mutation in 1989 and thereafter to develop therapies targeted at the mutation [4]. More recently, there have been two approved drugs that are given to patients based on their specific defect with the CFTR protein [5]. The field of oncology has also benefited, where current drug development is focused almost entirely on targeted therapy and immunologic approaches based on the influence of specific molecular drivers [6]. For example, the discovery of a chromosomal rearrangement between EML4 and ALK that results in a fusion protein with kinase activity and is present in approximately 4 percent of non-small cell lung cancers led to the development of crizotinib, a protein kinase inhibitor targeting ALK [7]. There are also examples of rare diseases that are highly heterogeneous, which may have less penetrant genes, such as Amyotrophic Lateral Sclerosis (ALS). Though multiple SNPs have been associated with ALS, a single gene has not been identified — yet [8].
The precision medicine approach may allow for improved clinical decision-making and facilitate the delivery of customized therapies that may be more effective than standard treatments. The benefits of precision medicine extend broadly from identifying high-risk individuals to enhancing prevention strategies and minimizing exposure to ineffective or toxic therapies. These effects all serve to reduce health care costs, optimize patient care, and improve patient quality of life [9]. Furthermore, research suggests that an understanding of personal genetic information increases patient compliance and participation in care [10,11].
Recognizing the value of the precision medicine approach, the U.S. government proposed the Precision Medicine Initiative in February 2015, promoting the use of genetic databases and advances in molecular biology, genomics, and bioinformatics in order to personalize health care [6]. The ability to practice precision medicine is largely due to the completion of the Human Genome Project (HGP), and the development of proteomics, metabolomics, genomics, and transcriptomics approaches to study biology and disease [1,6]. While genetic research was responsible for major breakthroughs in medicine prior to the completion of the HGP, the sequencing of the complete human genome has facilitated genetic research on a massive scale, fundamentally changing our understanding of all diseases, including those that did not have a strong heritable phenotype. Proteomics describes the analysis of proteins in a biological sample and is performed through a variety of methods. RNA sequencing is also available, thanks to the field of transcriptomics, which uses microarray analysis to assess cellular, genetic, and environmental effects on gene expression [1]. Metabolomics characterizes metabolic profiles by using mass spectrometry or magnetic resonance spectroscopy to assess how specific metabolites relate to different pathways [12]. In addition to whole genome sequencing, single-gene tests may be performed to evaluate risk for inherited diseases, the most notable example being BRCA testing for breast cancer. Disorders thought to have multi-factorial origins can be risk stratified using genetic panels, which sequence for mutations within different genes associated with a common phenotype [1]. It is further possible to predict a patient’s response to therapy, thanks to pharmacogenomic testing such as analyses of single nucleotide polymorphisms that alter the activity of the cytochrome P450 enzymes [13].
Challenges of Leveraging Precision Medicine for Rare Diseases
The “omics” technologies discussed above provide tools for elucidating the etiology and pathogenesis of rare diseases and identifying precision medicines for all patients with these rare diseases. However, there are many challenges that can get in the way of effectively leveraging these technologies for rare diseases, which can lack the large patient populations, coordinated army of scientific experts, and funding to generate data on a scale large enough to guide evidence-based practice. With 95 percent of rare diseases not having a single Food and Drug Administration (FDA)-approved therapy and few databases tracking treatments used, important clinical data is never captured and treating physicians are sometimes forced to make care decisions based on anecdotal judgments [14,15]. Accessing sufficient tissue samples for research is certainly one of the greatest challenges for rare disease research. Unfortunately, limited funding within the field can push researchers to work more independently rather than cooperatively. In some cases, researchers fight to keep their stored samples from being shared with other institutions. Also, it can be very difficult to generate sufficient data to win federal funding for diseases that are poorly understood, but scientific understanding of these diseases cannot be furthered without funding [14,16]. Finally, there are typically fewer experts able to take on major translational research projects and fewer pharmaceutical companies willing to fund rare disease studies. Of course, there are examples of rare diseases with exemplar disease organizations, such as the Cystic Fibrosis Foundation, Progeria Research Foundation, PXE (pseudoxanthoma elasticum) International, and Multiple Myeloma Research Foundation, which have successfully overcome these hurdles and proactively driven forward research. One rare disease that is confronting the above challenges head on is Castleman disease (CD).
Challenges for Castleman Disease Research
CD describes a heterogeneous group of disorders characterized by abnormal proliferation of morphologically benign lymphocytes due to excessive proinflammatory hypercytokinemia, particularly Interleukin-6 (IL-6) [17]. The three disorders are unicentric CD (UCD), Human Herpes Virus-8 (HHV-8) associated multicentric CD (HHV-8-associated MCD), and HHV-8-negative or idiopathic multicentric CD (iMCD) (Table 1). Together, there are an estimated 6,500 to 7,700 new cases diagnosed each year in the United States [18].
Table 1. Features of the different types of Castleman Disease.
| Type of Castleman Disease | Type of Lymphadenopathy | Pathology | IL6 driven inflammatory syndromea | Virologic Status | Treatment |
| Unicentric | Localized | 90% hyaline vascular | Typically not | Negative for HHV8 by QCPR or Negative LANA-1 stain | Complete Excision |
| Multicentric HHV8 positive | Generalized ±hepatosplenomegaly | plasmacytic or plasmablastic | Yes | Positive for HHV8 by QCPR; May be positive for HIV | Rituximab ±etoposide, Optional valganciclovir maintenance |
| Multicentric HHV8 negative | Generalized ±hepatosplenomegaly | Mostly plasmacytic, but can be hyaline vascular or mixed cellularity | Yes, but variable clinical presentation from mild to very severe and some cases do not respond to anti-IL-6 therapy | Negative for HHV8 by QCPR or Negative LANA-1 stain, Negative for HIV | Siltuximab, Tocilizimab, Rituximab, In severe cases Chemotherapy |
aSymptoms: fevers, night sweats, anorexia, weight loss, fatigue. Laboratory abnormalities: anemia, thrombocytopenia or thrombocytosis, elevated CRP, WESR, fibrinogen, hypergammaglobulinemia, abnormal renal function, increased IL6, VEGF, IL10.
Proliferation occurs in a single lymph node or region of lymph nodes in UCD, and surgical resection of the affected node(s) is usually curative [18]. Both HHV-8-associated MCD and iMCD are characterized by systemic lymphadenopathy, polyclonal lymphocyte and plasma cell proliferation, autoimmune manifestations, and multi-organ system dysfunction [17]. Clinically, patients may exhibit fevers, night sweats, weight loss, ascites, pleural effusions, and organomegaly in the setting of systemic inflammation and laboratory abnormalities [19]. In HHV-8-associated MCD, HHV-8 infects B-cells, evades host immunity (most often in immunocompromized patients), and replicates, producing lytic products that stimulate viral IL-6 and signal human IL-6 release, which provokes an inflammatory cascade [20]. Depletion of B-lymphocytes, which host HHV-8, with rituximab is effective in a large proportion of patients [21]. In HHV-8-negative iMCD patients, IL-6 and other proinflammatory cytokines also provoke an intense inflammatory cascade. However, the etiology, activated inflammatory pathways, diagnostic biomarkers, and pathological cell types are unknown [17].
No formal diagnostic criteria exist for any form of CD, and diagnosis is typically made by exclusion of other disorders that can cause similar clinical and histopathologic features [19]. Since the etiology and pathogenesis of iMCD have not been fully elucidated, a wide range of treatments are used with variable effectiveness, including cytotoxic chemotherapy, anti-IL-6 monoclonal antibodies, rituximab, immunomodulators, and steroids. Anti-IL-6 therapy is the only therapy studied in a randomized controlled trial, which demonstrated safety and efficacy for a significant proportion of subjects [22]. Despite progress for MCD, the largest and most recent case series found that approximately 35 percent of MCD patients died within 5 years [23].
Castleman Disease Collaborative Network
The Castleman Disease Collaborative Network (CDCN) was founded in 2012 to accelerate research and drug development for CD by facilitating collaboration among researchers and clinicians, investing funds in consensus-prioritized projects, and supporting patients, their families, and health care providers [24].
At the time of the CDCN’s creation, limited time and money were being invested into elucidating the etiology and pathogenesis of iMCD or UCD. The physicians and researchers interested in CD were using different sub-classification systems to describe the disease, and there was no consensus on diagnostic criteria. There was inaccurate information about epidemiology and prognosis from reputable medical sources. The previously proposed model of pathogenesis suggested that benign IL-6 secreting lymph node tumors were the source of the CD inflammatory storm even though all of the cells in the lymph node were polyclonal [17].
Prioritizing Precision Medicine Research
The CDCN began by first creating a global community of more than 300 researchers and physicians and assembling a Scientific Advisory Board (SAB) comprised of international CD experts. Based upon communication among members of the CDCN network and a systematic literature review, a new model of iMCD pathogenesis was published in Blood that suggests that the enlarged lymph nodes are reactive changes to the excess cytokines rather than the root cause of the cytokines [17]. The paper also proposed three hypotheses for what could be the mechanism underlying the cytokine release. To test these hypotheses, the CDCN’s SAB developed a consensus prioritized research agenda, termed the International Research Agenda (IRA). Projects prioritized by the IRA include serum proteomics studies (data currently being analyzed), a pathogen discovery study to identify specific viruses that may trigger iMCD using RNA sequencing (in process), flow cytometry and immunohistochemistry to identify pathological cell types involved in iMCD (in process), whole exome sequencing of germline samples (preparing to launch), and whole exome sequencing of CD lymph nodes to search for somatic mutations (in the queue). Since completing the IRA, the CDCN has been working to execute it by recruiting academic collaborators and contracting directly with contract research organizations (CRO). Fundraising is provided first to the projects with the highest near-term impact and the smallest relative financial cost, chiefly through the engagement of patients and loved ones to raise awareness within their own communities.
Unlike the typical approach that many disease research organizations take of first raising funds and then inviting researchers to apply to use the funding how they see fit (Figure 1), the CDCN built a global community, leveraged the community to prioritize research studies, recruited collaborators or CROs, and then raised funds to execute the community-prioritized research. The CDCN’s method enables limited funding and tissue samples to be used most efficiently, because the projects are determined based on the consensus of the Scientific Advisory Board rather than individual priorities and capabilities [24] (Figure 2).
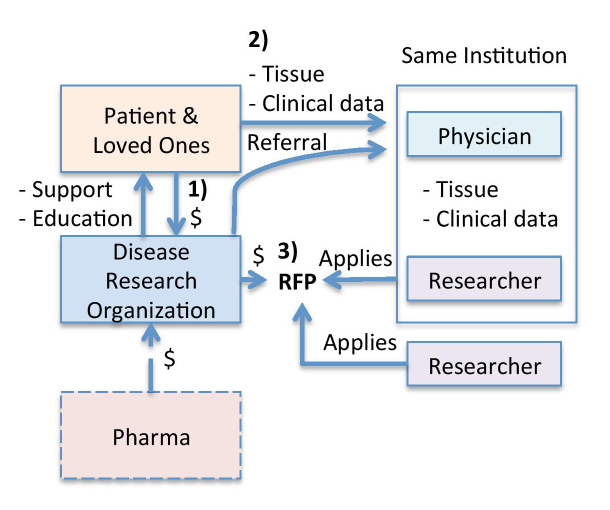
Figure 1.
Traditional research model. In this model, 1) Disease Research Organizations (DRO) first provide supportive resources to and raise money from patients and loved ones. 2) DROs also often refer patients to a few expert physicians, who collect clinical data and tissue samples. 3) With funding in hand, the DRO then places Request for Proposals (RFPs) and invites researchers to submit applications for funding. The researchers located at institutions that see the most patients clinically have access to essential tissue samples and clinical data, so they have the greatest likelihood of winning competitive grants. These individual researchers and the DRO grant review committee, thus, shape the research agenda for a given disease. Occasionally, pharmaceutical companies and governmental institutions fund DROs to distribute grants for research, but usually not until promising preliminary data has been generated.
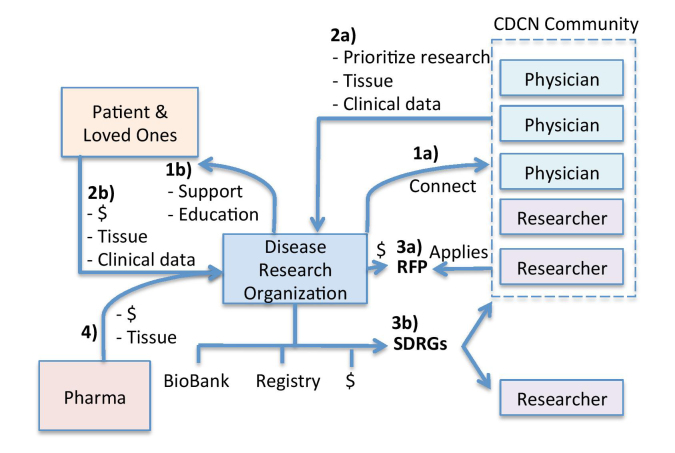
Figure 2.
CDCN’s research model. The CDCN has taken a four-step approach to drive forward “omics” research: 1a) The CDCN identified and connected the research community and (1b) provided supportive resources to patients and loved ones. Then, the CDCN turns to the research community (2a) and patient community (2b) to prioritize key research studies into an International Research Agenda as well as collect and centrally store clinical data and tissue samples. With the top priority projects identified, the CDCN solicits funding from patients and loved ones for specific projects. Then, the CDCN provides two mechanisms for funding: (3a) investigator-initiated research grants through traditional requests for proposals and (3b) strategically directed research grants where the DRO recruits the top experts within the community or outside of the community to do the studies. 4) Pharmaceutical companies are more eager to contribute tissue samples and funding for clearly defined projects with the greatest likelihood of return.
Linking Natural History with the Genome
The CDCN is in the process of creating a hybrid global patient registry and natural history study of CD, the ACCELERATE study. Accelerating Castleman Care with Electronic Longitudinal Registry, E-Repository, and Treatment Effectiveness Research (ACCELERATE) will be an observational, web-based registry that combines clinical data from patients internationally to aid in the understanding of CD and facilitate further research projects. Slated to begin enrollment in early 2016, ACCELERATE will generate data on the diagnosis, treatment, and clinical outcomes of CD [24]. The CDCN is also in the process of developing a biobank to streamline the collection, storage, and processing of blood and tissue samples from the CD community.
Precision Medicine for MCD
Currently, patients with MCD are treated distinctly based upon the presence or absence of HHV-8 in lymph node and blood samples. Patients infected with HHV-8 are typically treated with rituximab based on its demonstrated effectiveness and the basic understanding of B-lymphocytes hosting HHV-8 [20,21]. iMCD patients are treated with a variety of different treatments, including therapy targeting IL-6 with siltuximab or tocilizumab, rituximab, immunosuppresants, corticosteroids, and cytotoxic chemotherapy [25,26]. Siltuximab, an anti-IL-6 monoclonal antibody, became the first and only FDA- and EMA-approved therapy for iMCD in 2014 [27]. The approval was based on positive data from the only randomized controlled trial that has ever been performed for iMCD, which demonstrated significant symptomatic improvement and lymph node regression in 34 percent of patients on siltuximab versus 0 percent of patients on placebo [28]. The CDCN’s most urgent priority is uncover the molecular mechanisms of iMCD to determine what other therapies may be effective in combination with or as an alternative to siltuximab therapy in those that fail.
Discovery-level research approaches, including genomics, proteomics, and transcriptomics, will allow a precision medicine approach in iMCD by revealing biomarkers that suggest clinically relevant subsets of patients, predict impending disease activity, and/or predict which patients will best respond to the specific therapies available. As iMCD is a heterogeneous disease, diagnosis is nebulous, and the best available treatment approach for an individual patient is unknown. Identification of molecular subtypes of iMCD should allow for more accurate diagnosis and treatment, help with earlier intervention to prevent or reduce the severity of active disease, and generate more personalized information on disease course and outcomes.
Engaging the Community for Research
In addition to focusing on research priorities, engagement of the global physician, researcher, and patient community also has helped to facilitate research. The CDCN provides up-to-date information to community members and solicits feedback to build consensus among members regarding the IRA. Importantly, the CDCN’s culture of collaboration has enabled acquisition of clinically annotated samples from around the world for CDCN-funded research studies. Furthermore, the CDCN’s online community enables rapid communication and hypothesis generation. Also, CDCN leaders have completed a systematic literature review, which synthesizes information about clinical features of CD and specific responses to therapies [19], with the hope that this data can be used to identify patient groups that may respond better to one treatment than another (and corroborate these patient groups with “omics” data).
Conclusion and Outlook
It is not possible to advance understanding, research, and treatment for rare diseases like CD without highly structured, goal-directed, global collaboration. The inclusive, multi-faceted network provided by the CDCN is of tremendous value as it facilitates scientific discovery and supports scientists, who can often struggle to identify funding and tissue samples to test their hypotheses.
Precision medicine research facilitates discovery of novel treatments and has the potential to identify the specific patients who will benefit from these treatments [3]. Organizations that facilitate genomics and proteomics research have the potential to generate crucial data that will transform diagnosis and care of CD. In this respect, the CDCN’s infrastructure may serve as a model for other rare disease organizations to facilitate efficient, high-impact translational research that will improve outcomes for thousands of patients.
Abbreviations
- ALS
Amyotrophic Lateral Sclerosis
- CD
Castleman disease
- CDCN
Castleman Disease Collaborative Network
- CF
cystic fibrosis
- CRO
contract research organizations
- HGP
Human Genome Project
- HHV-8
Human Herpes Virus-8
- IL-6
Interleukin-6
- iMCD
idiopathic MCD
- IRA
International Research Agenda
- MCD
multicentric Castleman disease
- PXE
pseudoxanthoma elasticum
- SAB
Scientific Advisory Board
- UCD
unicentric CD
- FDA
Food and Drug Administration
- ACCELERATE
Accelerating Castleman Care with Electronic Longitudinal Registry, E-Repository, and Treatment Effectiveness Research
References
- Huser V, Sincan M, Cimino JJ. Developing genomic knowledge bases and databases to support clinical management: current perspectives. Pharmgenomics Pers Med. 2014;7:275–283. doi: 10.2147/PGPM.S49904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic and Rare Diseases Information Center (GARD). Frequently Asked Questions. National Institutes of Health [Internet] [cited 11 Aug 2015]. Available from: https://rarediseases.info.nih.gov/about-gard/pages/31/frequently-asked-questions .
- Litterman NK, Rhee M, Swinney DC, Ekins S. Collaboration for rare disease drug discovery research. F1000Res. 2014;3:261. doi: 10.12688/f1000research.5564.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B. et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Cutting GR. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat Rev Genet. 2015;16(1):45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Bang YJ, Kwak EL. et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;5(10):1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, McLeod HJ, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med. 2013;5(189):189sr4. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A, Visscher PM. Motor neuron disease: Common genetic variants and the heritability of ALS. Nat Rev Neurol. 2014;10(10):549–550. doi: 10.1038/nrneurol.2014.166. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Ginsburg GS, Van Nuys K, Agus D, Goldman D. Aligning incentives to fulfill the promise of personalised medicine. Lancet. 2015;385(9982):2118–2119. doi: 10.1016/S0140-6736(15)60722-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Strange C, Jones Y. et al. Does genetic testing result in behavioral health change? Changes in smoking behavior following testing for alpha-1-antitrypsin deficiency. Ann Behav Med. 2007;33(1):22–28. doi: 10.1207/s15324796abm3301_3. [DOI] [PubMed] [Google Scholar]
- Collins RE, Wright AJ, Marteau TM. Impact of communicating personalized genetic risk information on perceived control over the risk: a systematic review. Genet Med. 2011;13(4):273–277. doi: 10.1097/GIM.0b013e3181f710ca. [DOI] [PubMed] [Google Scholar]
- Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134(5):714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Evans WE, McLeod HL. Pharmacogenomics – drug disposition, drug targets, and side effects. N Engl J Med. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- Detterbeck FC. The creation of the international thymic malignancies interest group as a model for rare diseases. Am Soc Clin Oncol Educ Book. 2012:471–474. doi: 10.14694/EdBook_AM.2012.32.23. [DOI] [PubMed] [Google Scholar]
- Miyamoto B, Kakkis E. The potential investment impact of improved access to accelerated approval on the development of treatments for low prevalence rare diseases. Orphanet J Rare Dis. 2011;6:49. doi: 10.1186/1750-1172-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: A way out of a conundrum. BMJ. 1995;311:1621–1625. doi: 10.1136/bmj.311.7020.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924–2933. doi: 10.1182/blood-2013-12-545087. [DOI] [PubMed] [Google Scholar]
- Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman's disease: A systematic review of 404 published cases. Ann Surg. 2012;255(4):677–684. doi: 10.1097/SLA.0b013e318249dcdc. [DOI] [PubMed] [Google Scholar]
- Fajgenbaum D, Liu A, Ruth J, Nabel C, Finkelman B, Kurzrock R. et al. HHV-8-Negative, Idiopathic Multicentric Castleman Disease (iMCD): A Description of Clinical Features and Therapeutic Options through a Systematic Literature Review. Blood. 2014;124(21):4861. [Google Scholar]
- Suda T, Katano H, Delsol G. et al. HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman’s disease. Pathol It. 2001;51(9):671–679. doi: 10.1046/j.1440-1827.2001.01266.x. [DOI] [PubMed] [Google Scholar]
- Bower M, Newsom-Davis T, Naresh K. et al. Clinical features and outcome in HIV-associated multicentric Castleman's disease. J Clin Oncol. 2011;29(18):2481–2486. doi: 10.1200/JCO.2010.34.1909. [DOI] [PubMed] [Google Scholar]
- van Rhee F, Wong RS, Munshi N, Rossi JF, Ke XY, Fosså A. et al. Siltuximab for multicentric Castleman’s disease: a randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15(9):966–974. doi: 10.1016/S1470-2045(14)70319-5. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Armitage JO, Loe MJ. et al. The clinical spectrum of castleman’s disease. Am J Hematol. 2012;87(11):997–1002. doi: 10.1002/ajh.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleman Disease Collaborative Network. Annual Report. Philadelphia PA: 2015. [Google Scholar]
- Barquero N. Siltuximab: a new option for the management of Castleman’s disease. Drugs Today (Barc) 2015;51(1):21–28. doi: 10.1358/dot.2015.51.1.2234002. [DOI] [PubMed] [Google Scholar]
- Liu YC, Stone K, van Rhee F. Siltuximab for multicentric Castleman disease. Expert Rev Hematol. 2014;7(5):545–557. doi: 10.1586/17474086.2014.946402. [DOI] [PubMed] [Google Scholar]
- Deisseroth A, Ko CW, Nie L. et al. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin Cancer Res. 2015;21(5):950–954. doi: 10.1158/1078-0432.CCR-14-1678. [DOI] [PubMed] [Google Scholar]