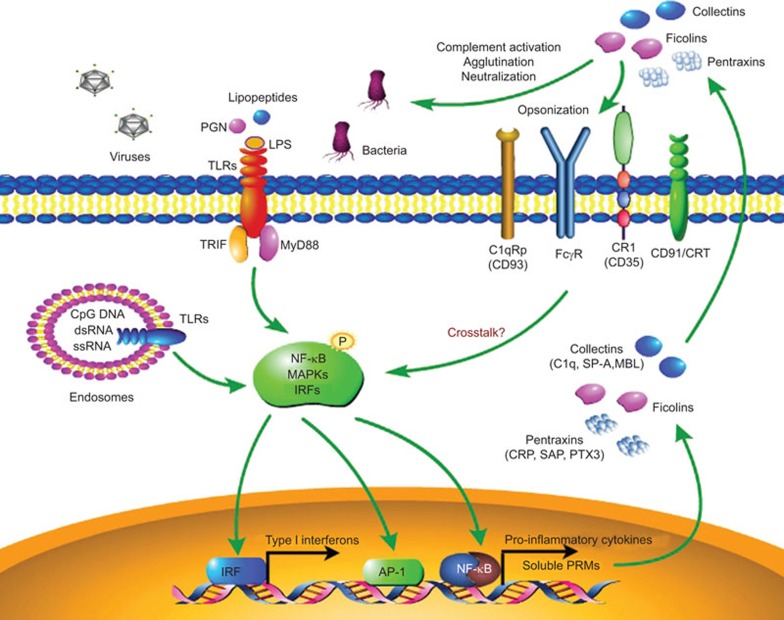
Figure 1.
Innate immune responses initiated by cellular TLRs and extracellular PRMs. Cellular TLRs and extracellular soluble PRMs are critical for sensing evading pathogens and triggering the innate immune responses. Pathogen-associated molecular patterns can be recognized by cellular TLRs in different cellular compartments. After ligation, TLRs can initiate downstream activation of NF-κB, MAPKs and IRFs through different signaling pathways that lead to the production of pro-inflammatory cytokines and type I interferon. Soluble PRMs are rapidly produced and secreted into the extracellular fluid in response to various danger signals and TLR engagement. Soluble PRMs generally belong to different molecular classes, including pentraxins (CRP, SAP, PTX3), collectins (C1q, SP-A, SP-D, MBL) and ficolins. These humoral PRMs primarily act through complement activation, agglutination and neutralization. They also have cellular receptors such as CR1 (CD35), CD91/CRT, C1qRp (CD93) and FcγR. After binding to these receptors, soluble PRMs can facilitate the recognition and phagocytosis of a microorganism by immune cells (opsonization) and can regulate the functions of cellular TLRs via mechanisms that remain unclear. CRP, C-reactive protein; IRF, IFN regulatory factor; MBL, mannan-binding lectin; MyD88, myeloid differentiation factor 88; PRM, pattern recognition molecule; SAP, serum amyloid P component; SP-A, surfactant protein A; TLR, Toll-like receptor; TRIF, Toll-IL-1 receptor domain-containing adaptor-inducing interferon-β.