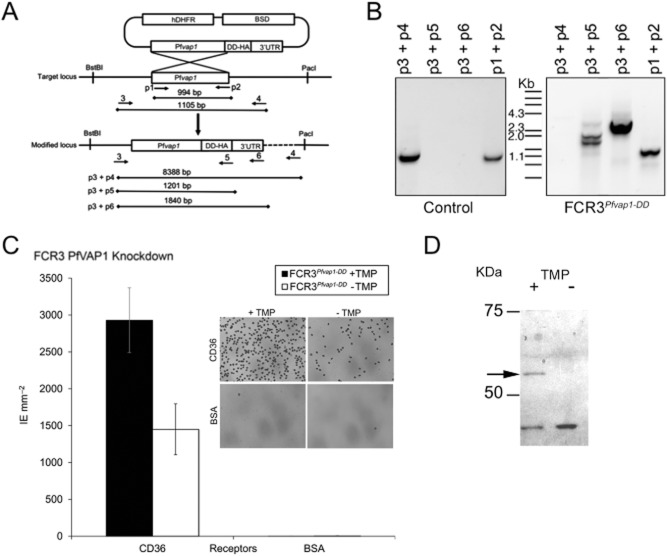
Fig. 4.
Knockdown of PfVAP1 reduces binding to CD36.A. Schematic representation of the knockdown (KD) strategy. Primer locations are indicated by arrows and numbered (e.g. p1). Expected amplicons are shown for the different primer pairs used.B. Integration of the PfVAP1-DD-HA in FCR3. Genomic DNA was used for PCR with the indicated primer pairs. Genomic DNA from parasites obtained from a control transfection with empty plasmid is shown.C. Binding of FCR3PfVAP1-DD KD. FCR3PfVAP1-DD KD parasites were cultured in the presence or absence of the drug TMP that stabilizes the degradation domain. A 50% reduction in binding was observed in FCR3PfVAP1-DD parasites without TMP. Mean binding of infected erythrocytes (IE) to purified CD36 and BSA is shown. Error bars = r standard deviation, n = 4 replicates). No effect of TMP on binding was detected using vector-transfected FCR3 parasites (data not shown). The inset shows Giemsa staining of FCR3PfVAP1-DD (+ /− TMP) bound to CD36 or the BSA negative control showing the effect of PfVAP1 in adhesion.D. Western blot of FCR3PfVAP1-DD parasites cultured in the presence (+) or absence (−) of TMP probed with anti-HA. A band of the expected molecular weight (52 kDA) corresponding the PfVAP1+HA fusion protein is observed in the presence of TMP that is lacking in the absence of TMP, confirming the degradation of the protein.