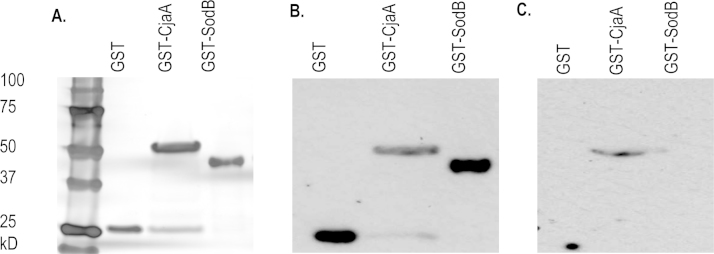
Fig. 1.
Purity and immunogenicity of vaccine preparations. (A) SDS–PAGE analysis of GST, GST–CjaA and GST–SodB vaccine preparations, followed by silver staining. (B) Western blot of the vaccine preparations in panel A using anti-GST antibody. (C) Western blot of the vaccine preparations in panel A using sera from Campylobacter-infected but non-vaccinated chickens. For all the lanes presented a total of 1 μg protein was used, in a final volume of 20 μl, mixed 1:1 with denaturing running buffer.