Abstract
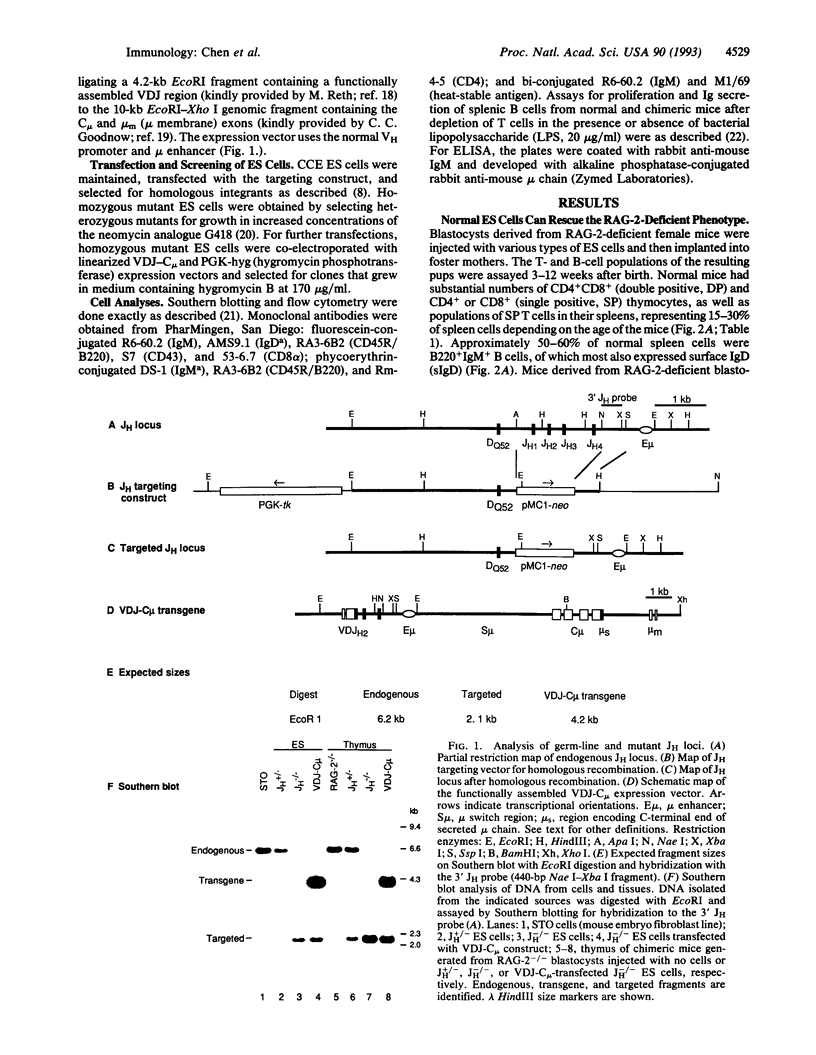
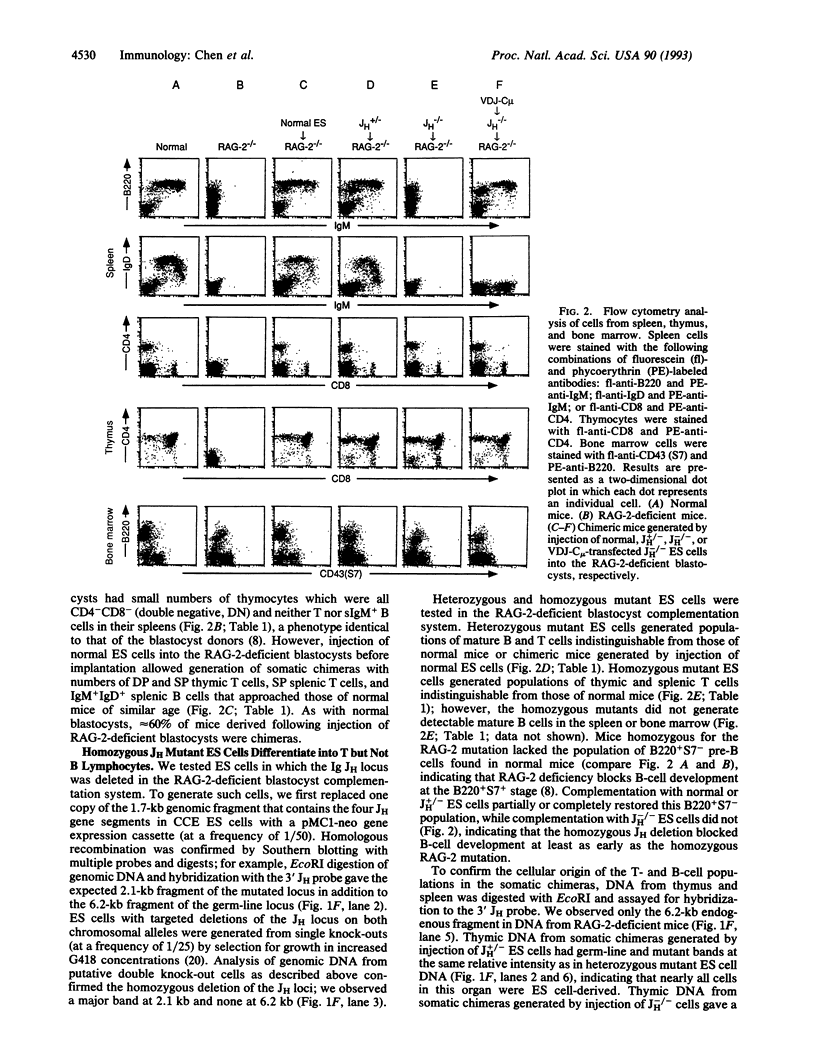
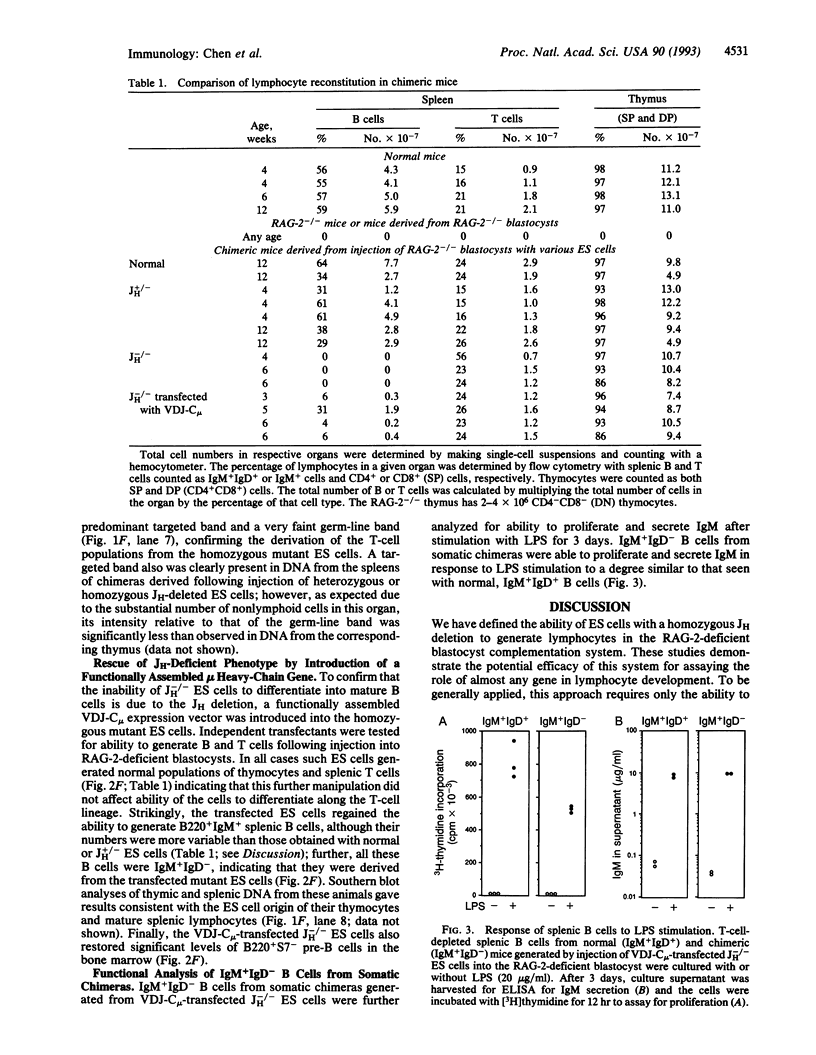
We describe a system to evaluate the function of lymphocyte-specific and generally expressed genes in the differentiation and/or function of lymphocytes. RAG-2 (recombination-activating gene 2)-deficient mice have no mature B and T lymphocytes due to the inability to initiate VDJ recombination. Blastocysts from RAG-2-deficient mice generate animals with no mature B and T cells following implantation into foster mothers. However, injection of normal ES cells into RAG-2-deficient blastocysts leads to the generation of somatic chimeras with mature B and T cells all of which derive from the injected ES cells (referred to as RAG-2-deficient blastocyst complementation). Complementation of RAG-2-deficient blastocysts with mutant ES cells heterozygous for a targeted mutation that deletes all immunoglobulin heavy-chain joining (JH) gene segments (JH+/-) also leads to generation of chimeras with normal B and T cells. However, complementation with ES cells homozygous for the JH mutation (JH-/-) generates animals with normal T cells but no B cells, due to a block in B-cell development at a very early stage. Transfection of a functionally assembled mu heavy-chain gene into the JH-/- ES cells prior to blastocyst injection rescues the JH-/- mutation and allows the generation of both mature T and mature B cells. The rescued B cells express IgM but not IgD and respond normally to bacterial lipopolysaccharide stimulation by proliferating and by secreting IgM.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Mechanism and developmental program of immunoglobulin gene rearrangement in mammals. Annu Rev Genet. 1989;23:605–636. doi: 10.1146/annurev.ge.23.120189.003133. [DOI] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Charron J., Malynn B. A., Fisher P., Stewart V., Jeannotte L., Goff S. P., Robertson E. J., Alt F. W. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992 Dec;6(12A):2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- Chen J., Trounstine M., Kurahara C., Young F., Kuo C. C., Xu Y., Loring J. F., Alt F. W., Huszar D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993 Mar;12(3):821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen U., Kosco M., Staerz U. Establishment and characterization of lymphoid and myeloid mixed-cell populations from mouse late embryoid bodies, "embryonic-stem-cell fetuses". Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2541–2545. doi: 10.1073/pnas.89.7.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement during pre-B cell differentiation. J Mol Cell Immunol. 1983;1(1):31–41. [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Field S. J., Johnson R. S., Mortensen R. M., Papaioannou V. E., Spiegelman B. M., Greenberg M. E. Growth and differentiation of embryonic stem cells that lack an intact c-fos gene. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9306–9310. doi: 10.1073/pnas.89.19.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W. P., Mak T. W. Embryonic stem cells and homologous recombination. Curr Opin Immunol. 1992 Apr;4(2):189–194. doi: 10.1016/0952-7915(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ramos J. C., Palacios R. In vitro differentiation of embryonic stem cells into lymphocyte precursors able to generate T and B lymphocytes in vivo. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9171–9175. doi: 10.1073/pnas.89.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Jones M., Goodnow C. C. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int Immunol. 1992 Feb;4(2):163–175. doi: 10.1093/intimm/4.2.163. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992 May;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Roes J., Rajewsky K. Cell autonomous expression of IgD is not essential for the maturation of conventional B cells. Int Immunol. 1991 Dec;3(12):1367–1371. doi: 10.1093/intimm/3.12.1367. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]



