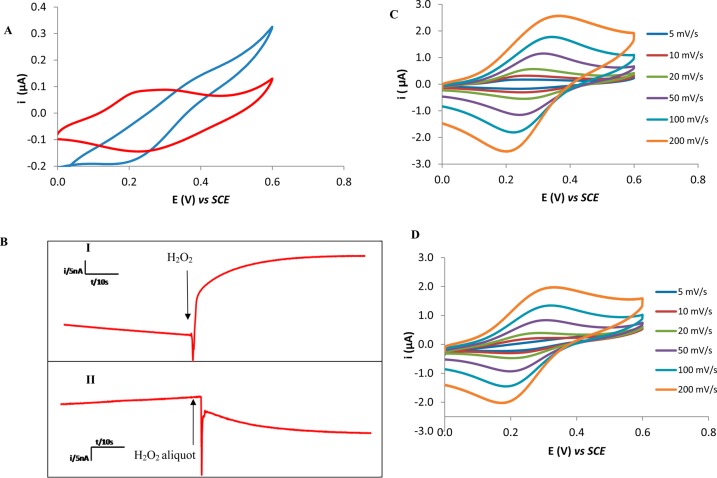
Figure 1.
(A) Typical cyclic voltammograms obtained in PBS (red) and 100 mM H2O2 (blue) at an ITO-SWCNTs-Osbpy electrode at a scan rate of 5 mV s–1. (B) Typical fixed potential amperograms obtained for addition of 1 mM aliquots of H2O2 to PBS using an ITO-SWCNT-Osbpy electrode poised 400 mV (I) to establish the presence of oxidative processes and at 150 mV (II) to investigate the reductive processes. (C, D) Typical cyclic voltammograms obtained for (C) PBS and in (D) 100 mM H2O2 solutions at ITO-SWCNT-Osbpy electrode at 5, 10, 20, 50, 100, and 200 mV s–1.