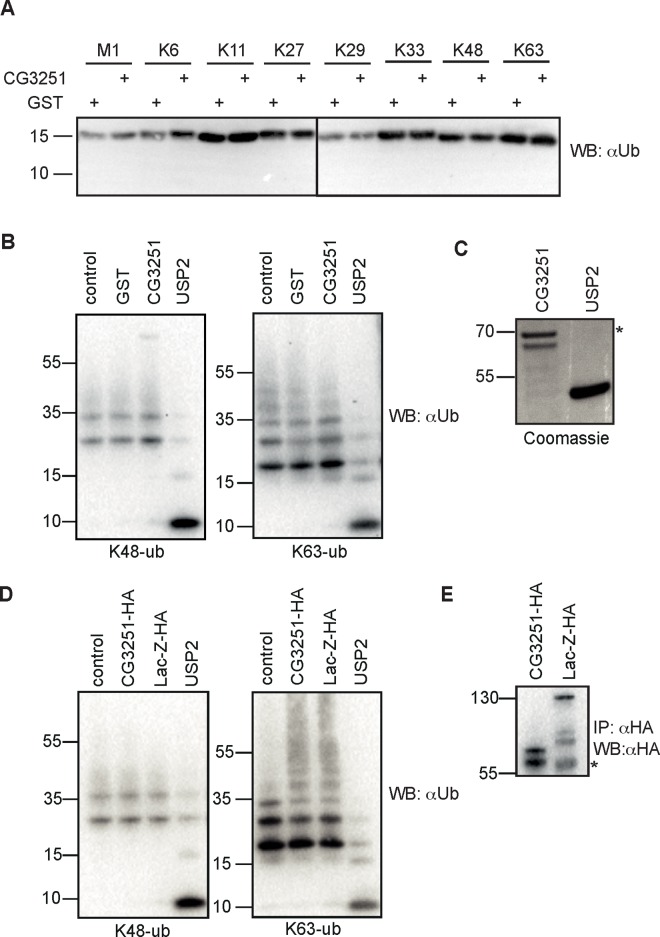
Fig 3. CG3251 does not process ubiquitin chains in vitro.
(A) Activity test of recombinant CG3251 protein on di-ubiquitin molecules. GST-CG3251 or GST protein as negative control was incubated with di-ubiquitin molecules of the indicated linkage types and cleavage assessed by anti-ubiquitin (Ub) Western Blot. None of the chain types was cut by CG3251. (B) Activity test on longer ubiquitin chains. K48- and K63-linked ubiquitin chains (3–7 ubiquitin units) were incubated with buffer only or recombinant proteins (GST, CG3251, USP2) and ubiquitin processing analyzed by WB. (C) Coomassie-stained gel to show input levels of CG3251 and USP2, respectively. * denotes a co-purified E. coli protein in the CG3251 preparation. (D) Drosophila S2 cells were transfected with expression constructs for HA-tagged CG3251 or HA-lacZ as control. Immunoprecipitated CG3251-HA or lacZ-HA was incubated with ubiquitin chains as in (B). Control sample was incubated with buffer only and recombinant USP2 was used as positive control. (E) anti-HA immunoprecipitates as used for cleavage reaction in D). * denotes the IgG heavy chain.