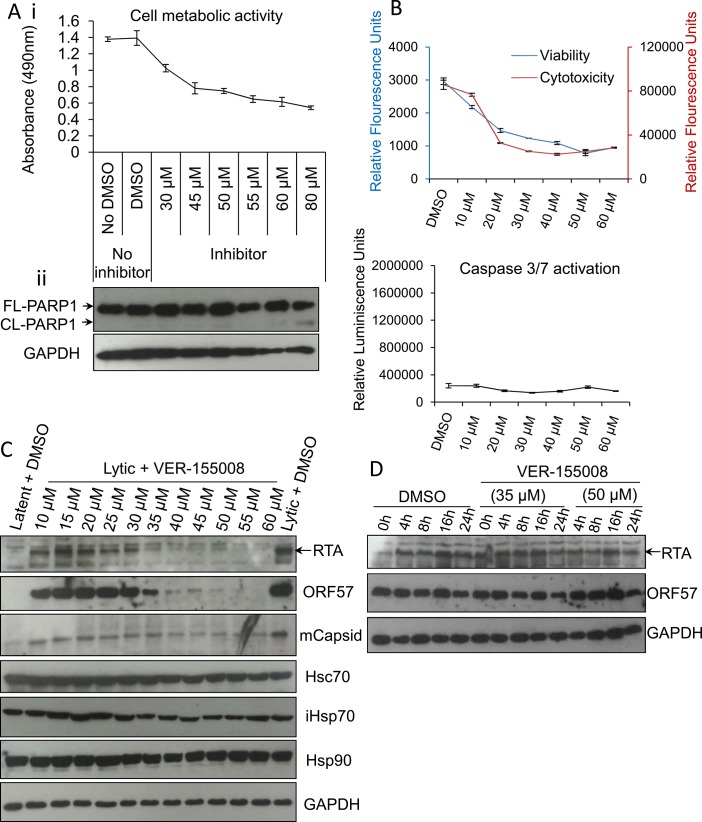
Fig 4. VER-155008 abrogated viral protein synthesis pre-translationally in HEK-293T rKSHV.219 cells.
(A) Cytotoxicity of VER-155008 was assessed in unreactivated cells exposed to increasing inhibitor concentrations for 24 h. (i) Cell metabolic activity was reduced in a dose-dependent manner as quantified using an MTS assay. (ii) Treatment with VER-155008 did not readily cause caspase 3-dependent cleavage of PARP1. Only at 80 μM cleaved (CL) PARP1 was observed faintly. (B) Unreactivated cells were exposed for 24 h to increasing inhibitor concentrations. A reduced number of metabolically active cells accompanied by no increased cytotoxicity was observed in the presence of the inhibitor. There was no activation of effector caspases even in the presence of 60 μM VER-155008. This phenotype is consistent with cell cycle arrest. Results were quantified with ApoTox-Glo Triplex Assay. (C) Immunoblot analysis showing that reactivated cells treated with 40 μM VER-155008 for 24 h resulted in abrogation of viral proteins compared with DMSO-treated samples while cellular proteins remained constant. (D) Cells were reactivated for 24 h to allow robust viral protein expression. Then, DMSO control (0.1%) or VER-155008 was added in conjunction with cycloheximide (CHX) at 100 μg/ml to block de novo protein synthesis. Protein lysates were collected at several times post-CHX addition (0, 4, 8, 16 and 24 h) and Western blotting was performed. As previously seen in TREx BCBL1-RTA cells, VER-155008 did not alter the half-life of RTA or ORF57 protein.