Figure 6.
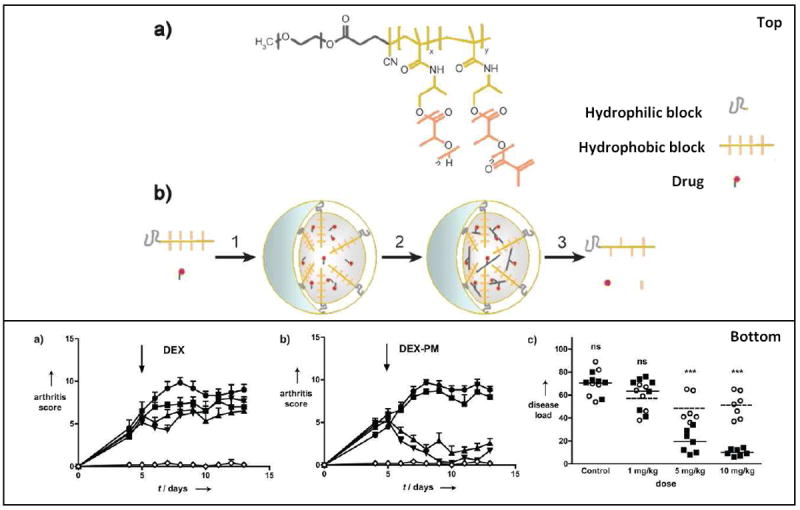
Results and micelle formulation from the study performed by Storm et Al. The top panel is a schematic representation of dexamethasoneloaded core-crosslinked polymeric micelles (DEX-PM). (a) Chemical structure of poly(ethylene glycol)-b-poly(N-(2hydroxypropyl)-methacrylamidelactate) (mPEG-b-pHPMAmLacn) block copolymers. (b) Illustrates the preparation, degradation, and drug release of DEX-PM. The bottom panels (a-b) highlight the results of their study. (a) and (b) show arthritis score after treatment. The mice received an i.v. injection of DEX (a) or DEX-PM (b) dosed at 1 mg/kg (■), 5 mg/kg (▲), or 10 mg/kg (▼). Control mice (●) received PBS or unloaded micelles. The disease load of each individual mouse upon treatment with free DEX (●) or DEX-PM (■). The disease load was defined as the area under the arthritis score curve from treatment (day 5) until the end of the study (day 13). The DEX-PM micelles provided a significant therapeutic effect and reduced both arthritis score and disease load compared to free DEX. Figure reproduced with permission from Wiley. [8]
