Abstract
Background. Infection with influenza A virus (IAV) increases susceptibility to respiratory bacterial infections, resulting in increased bacterial carriage and complications such acute otitis media, pneumonia, bacteremia, and meningitis. Recently, vaccination with live attenuated influenza virus (LAIV) was reported to enhance subclinical bacterial colonization within the nasopharynx, similar to IAV. Although LAIV does not predispose to bacterial pneumonia, whether it may alter bacterial transmigration toward the middle ear, where it could have clinically relevant implications, has not been investigated.
Methods. BALB/c mice received LAIV or phosphate-buffered saline 1 or 7 days before or during pneumococcal colonization with either of 2 clinical isolates, 19F or 7F. Middle ear bacterial titers were monitored daily via in vivo imaging.
Results. LAIV increased bacterial transmigration to and persistence within the middle ear. When colonization followed LAIV inoculation, a minimum LAIV incubation period of 4 days was required before bacterial transmigration commenced.
Conclusions. While LAIV vaccination is safe and effective at reducing IAV and coinfection with influenza virus and bacteria, LAIV may increase bacterial transmigration to the middle ear and could thus increase the risk of clinically relevant acute otitis media. These data warrant further investigations into interactions between live attenuated viruses and naturally colonizing bacterial pathogens.
Keywords: live attenuated influenza virus, middle ear bacterial colonization, bacterial transmigration, acute otitis media, pneumococcus, coinfection
Infection with influenza A virus (IAV) increases susceptibility to severe lower and upper respiratory tract (URT) bacterial infections, resulting in complications such as pneumonia, bacteremia, sinusitis, and bacterial acute otitis media (AOM) [1]; the latter is a major contributor to the global burden of pediatric disease and remains one of the most common diagnoses leading to the prescription of antimicrobial agents in the United States [2]. While bacterial AOM often occurs in isolation, increasing evidence suggests that primary or concurrent viral respiratory infections of the URT may play uniquely important roles in enhancing bacterial acquisition, colonization, and, ultimately, progression from asymptomatic bacterial carriage to AOM [3], notably from Streptococcus pneumoniae and Staphylococcus aureus [1, 4].
Although the mechanisms underlying influenza virus–mediated susceptibility to bacterial AOM are not entirely defined, they likely include a combination of IAV-mediated cytotoxic breakdown of mucosal and epithelial barriers of the URT [5–8] and aberrant innate immune responses to bacterial invaders in the immediate postinfluenza state, characterized by uncontrolled proinflammatory and antiinflammatory cytokine production, excessive leukocyte recruitment, and immunopathology [1, 9–13]. When coupled with diminished mucosal defenses, such an environment becomes increasingly hospitable for bacterial pathogens to flourish and cause clinical disease in the days and weeks following influenza virus infection.
Increasing evidence links the early innate immune response triggered by vaccination to long-term vaccine efficacy [14]. Thus, a goal of vaccination is to elicit an immune response as close to that elicited by the pathogen itself, without subsequent disease. The intranasally administered live attenuated influenza vaccine (LAIV) is composed of 1:1:6 reassortant viruses containing the hemagglutinin (HA) and neuraminidase (NA) surface proteins from wild-type viruses on a temperature-sensitive and attenuated backbone designed to enable efficient viral replication in the cooler temperatures of the URT but not the warmer temperatures of the lower respiratory tract (LRT) [15, 16]. Through selective replication in the URT, LAIV proteins are exposed to the host immune system in their native conformation, eliciting highly robust immunoglobulin A (IgA), serum immunoglobulin G (IgG), and cellular immune responses mimicking those of the pathogenic virus itself [17], without subsequent virus-mediated disease in the LRT [18, 19].
Recently, we demonstrated that LAIV, while safely providing long-term immunity against influenza and significantly reducing postinfluenza secondary bacterial infections [20], inadvertently enhances the duration and density of bacterial carriage in the nasopharynx of mice [21], a finding that has since been shown in humans [22]. Importantly, in contrast to wild-type IAV infections, LAIV did not alter bacterial outgrowth in the LRT and demonstrated no increases in the incidences of bacterial pneumonia or bacteremia. What is not known is whether LAIV virus replication in the URT may, like that of the wild-type IAV, inadvertently catalyze bacterial migration from the nasopharynx, where it is largely asymptomatic, into the middle ear, where it can increase the risk of symptomatic AOM [13, 23, 24]. Such an effect of the attenuated virus could result from LAIV-mediated inflammation of the epithelial cells of the pharyngotympanic tube [13] or from elevated bacterial density within the nasopharynx [25].
MATERIALS AND METHODS
Vaccinations and Infectious Agents
LAIV viruses were developed from a parent H3N2 1:1:6 reassortant virus developed as described previously [26]. The surface glycoproteins HA and NA were from the A/Hong Kong/1/68 (HK68) and A/Sydney/5/97 (Syd97) isolates, respectively, and the 6 internal protein gene segments were from A/Puerto Rico/8/34 or PR8 (referred to hereafter as WT virus). LAIV consisted of a temperature-sensitive (ts) attenuated variant of the WT virus (HK/Sydts) that contains site-specific mutations in the PB1 and PB2 RNA segments of the genome as described previously [26, 27]. These mutations are found in the attenuated A/Ann Arbor/6/60 master donor strain used to produce the commercial product known as FluMist (MedImmune, Gaithersburg, Maryland) [16]. LAIV viruses were propagated in 10-day-old embryonated chicken eggs at 33°C and quantitated in Madin–Darby canine kidney cells using the median tissue culture infective dose (TCID50). In vitro and in vivo growth dynamics have been reported elsewhere [21]. The pneumococcal carrier isolates ST425 (serotype 19F) and ST191 (serotype 7F) have been previously described [3]. These strains were engineered to express luciferase, as described elsewhere [3, 28].
Animal and Infection Models
Eight-week-old BALB/c mice (Jackson Laboratories, Bar Harbor, Maine) were used in all experiments. All inoculations were via the intranasal route. LAIV consisted of 2 × 106 TCID50 HK/Sydts virus in 40 µL of phosphate-buffered saline (PBS). Pneumococcal infections with 19F and 7F Streptococcus pneumoniae were as described previously [3]. Briefly, bacterial cultures were grown in Todd–Hewitt broth (Difco Laboratories, Detroit, Michigan) containing 0.5% yeast (THY) until mid- to late-log phase (OD, approximately 0.3,) and aliquots were stored at −80°C in 10% glycerol and quantified via serial dilution on blood agar plates. Inoculations were prepared from frozen aliquots and consisted of 1 × 106 and 1 × 105 colony-forming units of serotype 19F and 7F pneumococci, respectively, in 25 µL of PBS. Infections were initialized via careful administration of 12.5 µL of bacteria to each naris under general anesthesia with 2.5% inhaled isoflurane (Baxter Healthcare, Deerfield, Illinois). All experiments were conducted in biosafety level 2 facilities in a manner in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals.
Bioluminescent Imaging
Mice were imaged using an IVIS CCD camera (Xenogen) as described elsewhere [3]. Middle ear bacterial density was measured as total photons sec−1 cm−2 in prespecified regions covering the middle ear canal, and background (calculated for each mouse on a region of equal area over the hind limb) was subtracted. Positivity for bacteria within the middle ear was defined as a value of >40 000 photons sec−1 cm−2. This threshold has been previously described for this infection model, using the same instruments and laboratory environment [29]. Quantitation was performed using Living Image software (v. 3.0; Caliper Life Sciences) as described previously [3].
Although bioluminescent imaging of lux-expressing bacteria has previously been shown to be an efficient and accurate method for measuring bacterial density in the nasopharynx and lungs of mice and ferrets in vivo [3, 21, 29, 30], to ensure imaging was also appropriate for measuring bacterial presence and density within the middle ear, we compared values obtained from imaging to bacterial titers obtained by traditional methods. In short, the middle ear was dissected and completely homogenized in 1 mL of PBS, and serial dilutions were plated on 5% blood agar plates for quantification. Bacterial counts obtained from serial dilution plating were plotted against values obtained via IVIS just before dissection and showed strong linear correlation (R2 = 0.92; Supplementary Figure 1).
A single episode of bacterial middle ear colonization (MEC) was defined as any continuous detection of bacteria that was not interrupted by an interval of >2 days. This 2-day interval was important to account for normal fluctuations in bacterial density, whereby densities can temporarily fall below the threshold of detection (described above) without actually being cleared and then return to high levels. Additionally, episodes were categorized as early or late onset. Early onset was defined as an initial episode of MEC in a given mouse that occurred within 5 days of bacterial inoculation. Late onset was defined as any episode that commenced at least 2.5 days after clearance of a previous episode or at least 5 days after pneumococcal infection.
Statistical Analyses
All statistical analyses were performed within the R statistical computing environment (R, version 2.14; R Foundation for Statistical Computing, R Development Core Team, Vienna, Austria). Kaplan–Meier curves were constructed for freedom from MEC for each mouse per group, and the log-rank test was used to calculate statistically significant differences between groups. The frequency of MEC was plotted using Loess smoothing (span 0.2), and differences between daily frequencies in the vaccinated groups and those in the PBS controls were calculated using the Fisher exact test for differences in proportions. Differences in mean duration of MEC were calculated using 2-tailed 2-sample Students t tests. The false detection rate was used to adjust for multiple comparisons where appropriate, and statistical significance was considered when the calculated probability had an α level of <0.05.
RESULTS
LAIV Increases the Incidence of MEC in Mice Colonized With Pneumococci Before LAIV Receipt
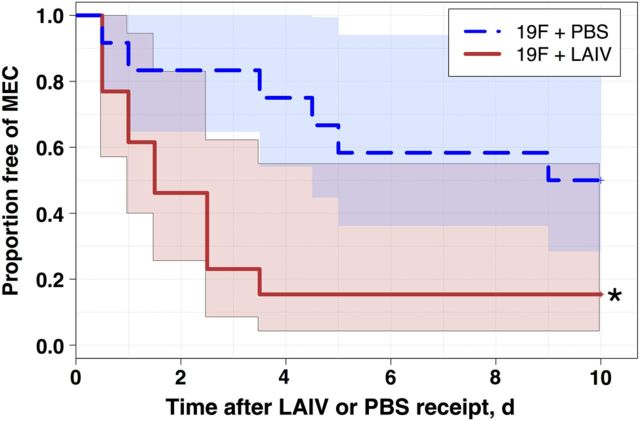
Nasopharyngeal carriage of pneumococcus is believed to be a prerequisite for MEC and subsequent pneumococcal AOM, and elevated bacterial density has been associated with transition from asymptomatic carriage to middle ear infections [25]. To determine whether LAIV vaccination of pneumococci-colonized mice may enhance bacterial transmigration to the middle ear, groups of 12–14 mice were colonized with serotype 19F pneumococcus (a clinical isolate often found colonizing the nasopharynx of children and a well-established model organism for colonization and AOM in mice [3]) 7 days before LAIV or PBS inoculation. A delay of 7 days was used because this was shown to be a sufficient interval over which bacteria reached stable colonization, as assessed via IVIS imaging of the nasopharynx and as previously reported [31]. Within 12 hours after LAIV inoculation, mice demonstrated an increased incidence of MEC (Figure 1), as determined by in vivo imaging of the middle ear (see “Materials and Methods” section). By day 4 after LAIV receipt, 85% of mice had at least 1 episode of MEC, compared with only 25% of PBS controls. In the majority of cases, initial onset of MEC in the LAIV group occurred within the first 4 days following vaccination, and freedom from MEC stabilized in both groups after day 5 (with the exception of a single new case in the PBS group, which was detected on day 9). By day 10 following LAIV or PBS inoculation, the incidence of MEC in LAIV recipients remained significantly greater than that in PBS controls (85% in LAIV recipients vs 50% in PBS controls; P = .017).
Figure 1.
Live attenuated influenza virus (LAIV) enhances the incidence of bacterial middle ear colonization (MEC) in precolonized mice. Groups of 12–14 8-week-old BALB/c mice were colonized intranasally with serotype 19F pneumococcal bacteria engineered to express luciferase. Seven days later, mice were inoculated with LAIV or phosphate-buffered saline (PBS) vehicle as a control. MEC was measured via in vivo imaging of the middle ear at 12-hour intervals for the first 2 days following LAIV or PBS receipt and daily thereafter. Initial onset of bacterial MEC was recorded for each mouse, and Kaplan–Meier survival curves were constructed. Data are reported as freedom from MEC after LAIV or PBS inoculation, and the log-rank test was used to determine statistically significant differences between groups. *P < .05, compared with PBS controls.
Antecedent Receipt of LAIV Predisposes to Bacterial Transmigration
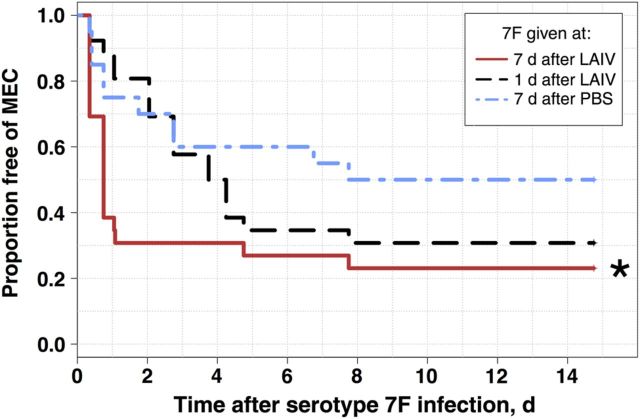
To address whether antecedent inoculation with LAIV predisposes to MEC after bacterial infection, and to ensure that the effect of LAIV on bacterial transmigration is not specific to serotype 19F pneumococci, mice received a colonizing dose of pneumococcal serotype 7F (a slightly more invasive clinical strain and a well-described model organism for pneumococcal AOM in mice [3]) at either 7 days or 1 day after LAIV receipt (n = 26 for each group) or 1 day after PBS receipt (n = 20; Figure 2). Inoculation with LAIV 7 days before pneumococcal infection led to immediate increases in the incidence of MEC, with only 30% (8 mice) remaining free from bacterial MEC 24 hours after infection; compared with 81% (21 mice) infected 1 day after LAIV receipt and 75% (15 mice) infected 1 day after PBS receipt. Following initial enhancement of MEC in mice infected 7 days after LAIV receipt, only 2 new cases (ie, cases in mice previously free from MEC) were seen over the following 2 weeks, at days 5 and 8 after bacterial infection.
Figure 2.
Freedom from middle ear colonization (MEC) following bacterial infection in recently vaccinated mice. Groups of 8-week-old BALB/c mice received live attenuated influenza virus (LAIV) 7 days (n = 26), LAIV 1 day (n = 26), or phosphate-buffered saline (PBS) 1 day (n = 20) before inoculation with serotype 7F pneumococcal bacteria engineered to express luciferase. In vivo imaging was used to detect bacterial MEC every 12–15 hours for the first 2 days following pneumococcal infection and at least daily thereafter. Initial onset of pneumococcal MEC was recorded for each mouse, and Kaplan–Meier survival curves were constructed to describe freedom from pneumococcal MEC. *P < .05, by the log-rank test, compared with PBS controls, corrected for multiple comparisons using the false-discovery rate.
An increased incidence of MEC was also detected in the group infected 1 day after LAIV receipt, but onset was distributed in this group, commencing between days 3 and 5 after infection, which corresponded to days 4–6 after LAIV receipt, a time previously demonstrated to maximize bacterial colonization of the nasopharynx [21].
LAIV-Mediated Enhancement of Bacterial Transmigration Is Delayed After Vaccination
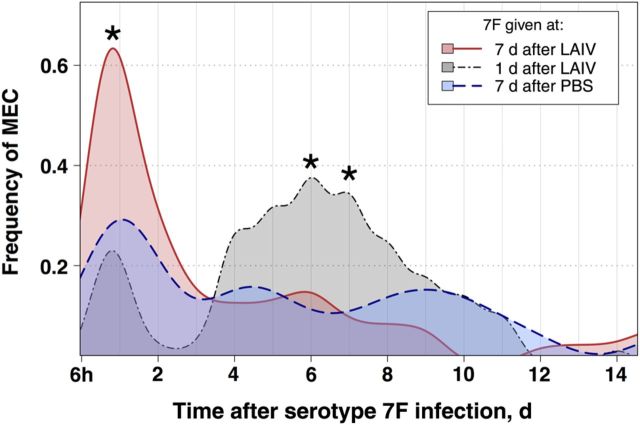
To better understand the dynamics of bacterial transmigration and MEC, we investigated the overall frequency per day of MEC for each group (Figure 3), which differs from our Kaplan–Meier analysis above in that the Kaplan–Meier analysis considers only time of first onset in a given mouse, rather than overall proportion with MEC at any particular time in our experimental groups. Consistent with the Kaplan–Meier analysis, mice vaccinated 7 days before pneumococcal infection had significantly increased frequencies of MEC for the first 24–48 hours after infection, compared with PBS controls. The frequency peaked in this group approximately 24 hours after infection, with slightly >60% (16 mice) with MEC. In contrast, only 20–30% of mice receiving LAIV or PBS 1 day before bacterial infection had evidence of MEC, and these episodes were very short lived, with almost no MEC in these groups by day 2. While the maximum frequency of MEC was reached 24 hours after infection in the group infected 7 days after LAIV receipt, mice infected only 1 day following LAIV receipt had a second wave of MEC episodes that began 4 days after LAIV receipt (Figure 3). This second wave of MEC, while lower in maximum frequency (approximately 40%) than in the group infected 7 days after LAIV receipt, had a broader and more sustained peak that lasted from day 4 to day 8 after bacterial infection.
Figure 3.
Frequency of middle ear colonization (MEC) following bacterial infection in recently vaccinated mice. Mice were inoculated with live attenuated influenza virus (LAIV) 7 days (n = 26), LAIV 1 day (n = 26), or phosphate-buffered saline (PBS) 1 day (n = 20) before infection with serotype 7F pneumococcus engineered to express luciferase, and in vivo imaging of the middle ear was performed to measure the presence of pneumococcal MEC. The frequency of MEC is plotted for each group, and differences in the daily frequency between groups were tested for statistical significance using the Fisher exact test for differences in proportions. *P < .05, compared with the PBS group.
LAIV Increases the Persistence of MEC
The duration of MEC was measured for each episode per mouse, as defined above, and mean durations were calculated for each group. The duration was significantly increased across all vaccinated groups, regardless of pneumococcal strain (ie, serotype 19F or 7F) or whether LAIV was given before or following pneumococcal infection. When LAIV or PBS was administered to mice with preestablished serotype 19F colonization, bacteria persisted in the middle ears nearly 2-fold longer than in PBS controls (2.3 vs 1.2 days; P < .05; Figure 4A). Similarly, when mice received LAIV 7 days or 1 day before bacterial infection, the mean durations of MEC episodes were 3-fold and 2-fold greater, respectively, than those for PBS controls (P < .05 for each comparison; Figure 4B). Interestingly, when episodes were classified into early and late onset (see “Materials and Methods” section for classification criteria), durations of early onset cases in the group infected 7 days after LAIV receipt were almost identical to durations of late-onset cases in the group infected 1 day after LAIV receipt (approximately 3.75 days in each group) and, in each case, the duration was >2-fold greater than that for their respective PBS controls (approximately 1.5 days; P < .05; Figure 4C). Alternatively, the duration of early onset episodes in the group infected 1 day after LAIV receipt and the duration of late-onset episodes in the group infected 7 days after LAIV receipt were no different than for PBS controls. Taken together with the findings of Kaplan–Meier analyses described above, these data demonstrate a strong influence of time since LAIV inoculation, rather than time since bacterial infection, with a minimum of 4 days after vaccination required before enhanced bacterial transmigration to and colonization of the middle ear is detected.
Figure 4.
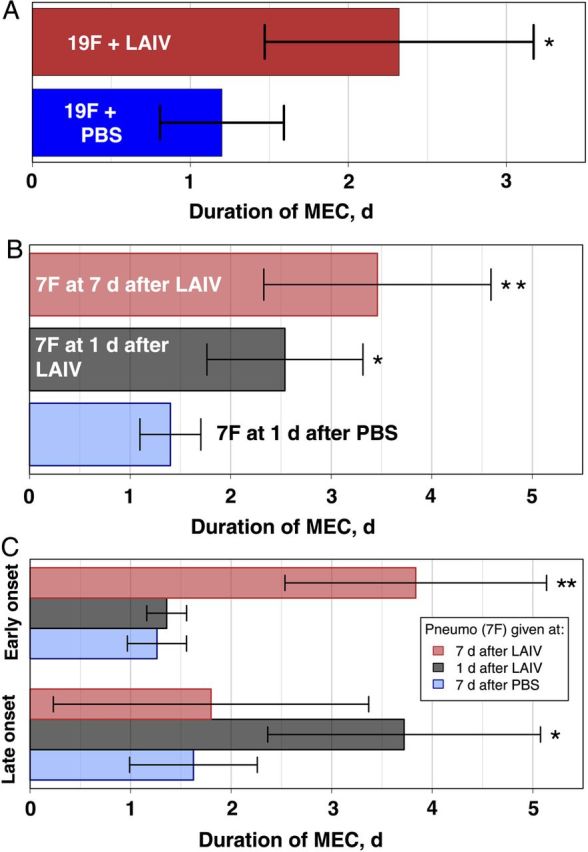
Live attenuated influenza virus (LAIV) enhances persistence of middle ear colonization (MEC). A and B, Groups of mice were colonized with serotype 19F pneumococcus 7 days before inoculation with LAIV (n = 14) or phosphate-buffered saline (PBS; n = 12; A) or received LAIV 7 days (n = 26), LAIV 1 day (n = 26), or PBS 1 day (n = 20) before infection with serotype 7F pneumococcus (B). The durations of MEC episodes were measured, and mean durations reported for serotype 19F (A) and serotype 7F (B) MEC, in which a single episode was defined as any continuous detection in a given mouse that was not interrupted by >2 days. C, Episodes of serotype 7F MEC were further classified as early onset (onset within the first 5 days following infection) or late onset (>2 days following termination of an early episode or >5 days after infection), and mean durations reported for each group. Statistically significant differences (vs PBS controls) were tested using 2-tailed 2-sample Student t tests with correction for multiple comparisons, using the false-discovery rate. Error bars represent 95% confidence intervals around the mean. *P < .05, **P < .001.
DISCUSSION
The potent and often lethal effects of a previous influenza virus infection on secondary pneumococcal invasive disease and pneumonia have been reported [1, 11, 32–34]. Viral replication induced epithelial and mucosal degradation, and the ensuing innate immune response yield diminished capacity to avert secondary bacterial infections. Recent clinical and experimental data suggest that influenza viruses may exert their influence, beginning in the URT, by enhancing susceptibility to bacterial colonization [3, 30, 35], increasing nasopharyngeal carriage density [23], and enhancing the incidence of AOM [13].
Although LAIV, in the longer-term, thwarts influenza virus and bacterial coinfections by inhibiting the viral infection [18, 31], LAIV vaccines have recently been found to enhance the density and duration of bacterial colonization within the nasopharynx of mice, and evidence has also been put forth for humans [21, 22, 36]. Importantly, unlike WT IAV, LAIV did not result in increased bacterial proliferation or disease in the LRT, presumably because of the temperature-sensitive nature of LAIV viruses, abrogating viral growth within the warmer temperatures of the lungs. Although LAIV did not effect clinical bacterial LRT infections, the effects of LAIV on transition from colonization to bacterial disease within the URT, a region where LAIV replicates efficiently, had not been studied.
Here, we found that vaccination with a mouse-adapted LAIV significantly increased bacterial transmigration to the middle ear and the duration of MEC, irrespective of bacterial serotype or order of viral versus bacterial inoculation. Interestingly, a minimum period of approximately 4 days was required before enhancement in pneumococcal transmigration and MEC was noted, when LAIV preceded pneumococcal infection.
The dynamics of increased MEC, with regard to time since vaccination, closely match increased pneumococcal colonizing dynamics of the nasopharynx following WT IAV or LAIV virus [21, 31] and support the notion that nasopharyngeal colonizing density may be associated with progression to AOM. Interestingly, the delay in increased onset of migration and MEC in mice vaccinated only 1 day before bacterial inoculation was approximately the same as the time to peak LAIV viral titers in the URT [21]. Thus, a majority of excess MEC occurs during or soon after viral clearance from the URT. This finding supports numerous reports [1, 10–12, 23] that point toward a complex coupling of poorly coordinated antibacterial innate immune defenses and epithelial damage following influenza virus infection, underlying the excess susceptibility to bacterial disease after influenza virus infection.
On the other hand, the steady increase in onset of MEC measured immediately following LAIV vaccination in serotype 19F–precolonized mice suggests that introduction of LAIV virus in the presence of existing bacterial colonization yields enhanced MEC that is concurrent with viral replication and precedes viral-mediated enhanced nasopharyngeal colonization, which tend to increase beginning on day 4 after LAIV inoculation. This suggests that the mechanisms of virus-induced bacterial AOM may differ according to order of inoculation. Indeed, it may be that even low levels of viral replication in the URT, while not immediately affecting overall bacterial carriage density in the nasopharynx, may rapidly disrupt a delicate balance that naturally exists to prevent asymptomatic carriage from transitioning to bacterial AOM.
It must be clearly emphasized here that any animal study, particularly mouse studies [37], must be viewed in light of the many caveats that exist when extrapolating findings from animal studies to humans. Although animal studies have been integral to our understanding of infectious diseases (and many other biological systems), the individual processes and dynamics often differ between the animal model—mice, in this case—and the human system, as has been shown [37].
While our data suggest that LAIV may enhance pneumococcal transmigration into the middle ear, it is clear that the overall effect of LAIV measured in humans has been that of significant reductions in viral influenza infections and otitis media [38]. While our data suggest a potential effect of LAIV to increase bacterial transmigration to the middle ear, a lack of detection in numerous large clinical trials in humans suggests that any effect is largely subclinical. As well, LAIV-mediated protection from primary influenza virus infections significantly reduces the opportunity for worse secondary bacterial infections [20], further reducing the incidence of LRT and URT bacterial disease, including bacterial AOM.
While we are confident that the overall effects of LAIVs are beneficial to reduce all-cause AOM across populations, as has been reported [39], our data here and previous reports [21] suggest a need for future investigations to more closely evaluate the effects of LAIV on bacterial respiratory pathogen dynamics, including unintended beneficial effects [20]. Indeed, as medicine becomes increasingly personalized [40], it may become possible to tailor classes of vaccines and avenues of vaccine delivery to the individual. In this particular example, considering the benefits of LAIV over inactivated injectable influenza vaccines [41], one could envision that the choice between a killed injectable vaccine and an intranasal LAIV might incorporate the risk of pneumococcal carriage or acquisition (based on factors such as the number of children in the household, the age of the vaccine recipient, and proximity to immunocompromised individuals) as a potential variable in the decision-making process.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by ALSAC–St. Jude Children's Research Hospital (to J. A. M. and J. W. R.) and the Burrough's Wellcome Fund (Molecules to Mankind fellowship to M. J. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009; 302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis 2010; 202:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008; 122:805–11. [DOI] [PubMed] [Google Scholar]
- 5.Hers JF, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet 1958; 2:1141–3. [DOI] [PubMed] [Google Scholar]
- 6.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis 1986; 134:1040–4. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Kurono Y, Ichimiya I, Suzuki M, Mogi G. Effects of influenza A virus on lectin-binding patterns in murine nasopharyngeal mucosa and on bacterial colonization. Otolaryngol Head Neck Surg 1999; 121:616–21. [DOI] [PubMed] [Google Scholar]
- 8.Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Med Microbiol Immunol 2013; 202:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahangian A, Chow EK, Tian X, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 2009; 119:1910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 2011; 186:1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 2008; 14:558–64. [DOI] [PubMed] [Google Scholar]
- 12.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol 2004; 172:7603–9. [DOI] [PubMed] [Google Scholar]
- 13.Short KR, Reading PC, Brown LE, et al. Influenza-induced inflammation drives pneumococcal otitis media. Infect Immun 2013; 81:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol 2009; 9:741–7. [DOI] [PubMed] [Google Scholar]
- 15.Chan W, Zhou H, Kemble G, Jin H. The cold adapted and temperature sensitive influenza A/Ann Arbor/6/60 virus, the master donor virus for live attenuated influenza vaccines, has multiple defects in replication at the restrictive temperature. Virology 2008; 380:304–11. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Lu B, Zhou H, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 2003; 306:18–24. [DOI] [PubMed] [Google Scholar]
- 17.Ambrose CS, Luke C, Coelingh K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respi Viruses 2008; 2:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol 2011; 186:987–93. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, Zengel JR, Suguitan AL, Jr, et al. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 2013; 208:594–602. [DOI] [PubMed] [Google Scholar]
- 20.Mina MJ, Klugman KP, McCullers JA. Live attenuated influenza vaccine, but not pneumococcal conjugate vaccine, protects against increased density and duration of pneumococcal carriage after influenza infection in pneumococcal colonized mice. J Infect Dis 2013; 208:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mina MJ, McCullers JA, Klugman KP. Live Attenuated Influenza Vaccine Enhances Colonization of Streptococcus pneumoniae and Staphylococcus aureus in Mice. mBio 2014; 5:e01040–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mina MJ, Klugman KP. Reply to “no clinical association of live attenuated influenza vaccine with nasal carriage of bacteria or acute otitis media”: specific recommendations for future studies. mBio 2014; 5:e01173–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 2011; 121:3657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 2013; 4:e00438–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Vaughan H, Byun R, Nadkarni M, et al. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord 2006; 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber VC, Thomas PG, McCullers JA. A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine 2009; 27:1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin H, Zhou H, Lu B, Kemble G. Imparting temperature sensitivity and attenuation in ferrets to A/Puerto Rico/8/34 influenza virus by transferring the genetic signature for temperature sensitivity from cold-adapted A/Ann Arbor/6/60. J Virol 2004; 78:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis KP, Yu J, Bellinger-Kawahara C, et al. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun 2001; 69:3350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosch JW, Iverson AR, Humann J, et al. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med 2014; 6:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diavatopoulos DA, Short KR, Price JT, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J 2010; 24:1789–98. [DOI] [PubMed] [Google Scholar]
- 31.Mina MJ, Klugman KP, McCullers JA. Live attenuated influenza vaccine, but not pneumococcal conjugate vaccine, protects against increased density and duration of pneumococcal carriage after influenza infection in pneumococcal colonized mice. J Infect Dis 2013; 208:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Short KR, Habets MN, Hermans PW, Diavatopoulos DA. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol 2012; 7:609–24. [DOI] [PubMed] [Google Scholar]
- 33.Nelson GE, Gershman KA, Swerdlow DL, Beall BW, Moore MR. Invasive pneumococcal disease and pandemic (H1N1) 2009, Denver, Colorado, USA. Emerg Infect Dis 2012; 18:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med 2007; 57:82–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Stol K, Diavatopoulos DA, Graamans K, et al. Inflammation in the middle ear of children with recurrent or chronic otitis media is associated with bacterial load. Pediatr Infect Dis J 2012; 31:1128–34. [DOI] [PubMed] [Google Scholar]
- 36.Thors V, Morales-Aza B, Christensen H, Vipond B, Muir P, Finn A. Live attenuated intranasal vaccine does not increase rates of nasal pneumococcal colonization in healthy children but may contribute to increased density. ISPPD-9 http://isppd.meetingxpert.net/swf/poster_viewer.aspx, 2014. Accessed 7 May 2014.
- 37.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–96. [DOI] [PubMed] [Google Scholar]
- 39.Heikkinen T, Block SL, Toback SL, Wu X, Ambrose CS. Effectiveness of intranasal live attenuated influenza vaccine against all-cause acute otitis media in children. Pediatr Infect Dis J 2013; 32:669–74. [DOI] [PubMed] [Google Scholar]
- 40.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–4. [DOI] [PubMed] [Google Scholar]
- 41.Tarride JE, Burke N, Von Keyserlingk C, O'Reilly D, Xie F, Goeree R. Cost-effectiveness analysis of intranasal live attenuated vaccine (LAIV) versus injectable inactivated influenza vaccine (TIV) for Canadian children and adolescents. Clinicoecon Outcomes Res 2012; 4:287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.