Abstract
Aims
The REGENERATE-DCM trial is the first phase II randomized, placebo-controlled trial aiming to assess if granulocyte colony-stimulating factor (G-CSF) administration with or without adjunctive intracoronary (IC) delivery of autologous bone marrow-derived cells (BMCs) improves global left ventricular (LV) function in patients with dilated cardiomyopathy (DCM) and significant cardiac dysfunction.
Methods and results
Sixty patients with DCM and left ventricular ejection fraction (LVEF) at referral of ≤45%, New York Heart Association (NYHA) classification ≥2 and no secondary cause for the cardiomyopathy were randomized equally into four groups: peripheral placebo (saline), peripheral G-CSF, peripheral G-CSF and IC serum, and peripheral G-CSF and IC BMC. All patients, except the peripheral placebo group, received 5 days of G-CSF. In the IC groups, this was followed by bone marrow harvest and IC infusion of cells or serum on Day 6. The primary endpoint was LVEF change from baseline to 3 months, determined by advanced cardiac imaging. At 3 months, peripheral G-CSF combined with IC BMC therapy was associated with a 5.37% point increase in LVEF (38.30% ± 12.97 from 32.93% ± 16.46 P = 0.0138), which was maintained to 1 year. This was associated with a decrease in NYHA classification, reduced NT-pro BNP, and improved exercise capacity and quality of life. No significant change in LVEF was seen in the remaining treatment groups.
Conclusion
This is the first randomized, placebo-controlled trial with a novel combination of G-CSF and IC cell therapy that demonstrates an improvement in cardiac function, symptoms, and biochemical parameters in patients with DCM.
Keywords: Heart failure, Dilated cardiomyopathy, Stem cells, Granulocyte colony-stimulating factor
See page 3070 for the editorial comment on this article (doi:10.1093/eurheartj/ehv515)
Introduction
Non-ischaemic dilated cardiomyopathy (DCM) is a leading cause of heart failure and the most common indication for transplantation worldwide.1,2 The prevalence of DCM is estimated at 1 in 2500 and although a proportion of patients recover cardiac function, the majority suffer a progressive decline in left ventricular ejection fraction (LVEF)3 with high levels of morbidity and mortality despite optimal medical care.4
Novel approaches to promote recovery of myocardial function in DCM have included cytokine and cell therapies. Clinical investigation of cytokine therapy alone has been limited and has failed to deliver long-lasting improvements in cardiac function.5 Autologous bone marrow-derived cell (BMC) therapy has moved rapidly from proof of concept in preclinical experiments to clinical trials of cardiac repair in the most part in patients with acute myocardial infarction or heart failure secondary to ischaemic heart disease. Although these early Phase I/II clinical trials have demonstrated mixed results, meta-analysis has suggested that autologous cell therapy with or without adjunctive cytokine has therapeutic potential in these patient groups.6 However, improvements in the intermediate outcome measures used in these trials has been modest, suggesting that adjunctive or alternative types of cell therapy may be needed to achieve clinically meaningful results.
Patients with DCM are a small proportion of those entered into these early stage trials. Two recent meta-analyses of autologous cell therapy in patients with DCM have shown beneficial effects on intermediate outcomes of disease such as cardiac function.7,8 To date, there are few trials in DCM that combine cytokine and progenitor cell injection none of which are blinded or fully controlled.9 Here we report the results of the first, randomized, blinded (within arm), placebo-controlled trial combining autologous cell therapy with adjunctive cytokine—granulocyte colony-stimulating factor (G-CSF)—in the treatment of patients with DCM. We hypothesized that intracoronary (IC) autologous BMC administration would augment the pleotropic effects of G-CSF on cardiac function leading to an increase in LVEF at 3 months compared with baseline accompanied by improvement in symptoms and biochemical markers of heart failure.
Methods
Study design and participants
The study is a randomized, single centre, placebo-controlled Phase II trial to determine if the administration of G-CSF alone or with adjunctive IC autologous BMC in patients with DCM leads to an improvement in LVEF. The trial was approved by an independent ethics committee, the Medicines and Healthcare Products Regulatory Agency, registered at approved registries (ClinicalTrials.gov: NCT01302171, EudraCT: 2009-013112-12) and was performed in accordance with the Declaration of Helsinki (1993) and the principles of the International Conference of Harmonization—Good Clinical Practice guidelines. The full protocol is available as Supplementary material online, Appendix and is summarized as follows.
Potential patients were assessed for recruitment after referral from heart failure specialists at the London Chest Hospital, the Heart Hospital London, and the Royal Brompton Hospital London. All trial procedures were carried out at the London Chest Hospital. Inclusion criteria were a diagnosis of non-ischaemic DCM with no secondary cause found, an LVEF of <45% (assessed by echocardiography at referral), symptoms classed as New York Heart Association (NYHA) 2 or greater and on optimal medical treatment (established for at least 6 months). Secondary cardiomyopathy was defined as pathological myocardial involvement associated with systemic disorders, e.g. endocrine and metabolic disorders, alcohol and drug toxicity, infiltrative disorders, and neuromuscular diseases.
Randomization and masking
After consenting for the trial, patients were randomized using a dedicated trial software system (IHD Clinical Bishops Stortford, Herts, UK) in a 1 : 1 : 1 : 1 simple randomization to one of four groups. The four groups included: the ‘peripheral placebo group’ who received peripheral subcutaneous injected saline, the ‘peripheral G-CSF group’ who received subcutaneous G-CSF (Granocyte™, Chugai Pharmaceutical UK Ltd, Mulliner House, London) (10 µg/kg/day) for 5 days, the ‘IC BMC group’ who underwent bone marrow harvest after 5 days of G-CSF and received IC infusion of autologous BMC, and the ‘IC serum group’ who also underwent bone marrow harvest after 5 days of G-CSF but received IC infusion of serum only. Intracoronary injection was standardized to deliver cells equally between the major epicardial vessels via the stop flow method as previously described.10 It was not possible for the study to be blinded across all four groups due to the invasive nature of the IC arm. However, participants and investigators were blinded within the IC arm between the IC BMC group and IC serum groups and in the peripheral arm between saline and G-CSF. Data analysers were entirely masked to group assignment in both trial arms.
Endpoints and definitions
The primary endpoint was the change in global LVEF at 3 months compared with baseline as assessed by advanced cardiac imaging. Secondary imaging endpoints included change in LVEF at 1 year (compared with baseline) and changes in LV volumes and myocardial mass from baseline at 3 months and 1 year. Secondary endpoints also included change in NT-proBNP levels, exercise capacity (VO2 peak) NYHA classification and quality of life as assessed by European Quality of Life-5 Dimensions (EQ5D), and Kansas City Cardiomyopathy Questionnaire (KCCQ) at 3 months and 1 year compared with baseline. Mortality and adverse cardiovascular events (MACE) defined as all-cause death, myocardial infarction, hospitalization for heart failure, or major arrhythmias were assessed at 3 months and 1 year. The safety of the IC infusion was assessed by measurement of creatine kinase (CK) and Troponin T concentrations at 12 h post infusion and procedural complications.
Advanced cardiac imaging
Cardiovascular magnetic resonance (CMR) or cardiac computed tomography (CT) for those unable to undergo CMR were performed at baseline and 3 months. Conformity of the imaging modality was assessed separately to ensure reproducibility and sensitivity. The standard error of measurement of MRI and CT was 1.93 and 2.3%, respectively. Multi-phase cardiac datasets with full left ventricular coverage were acquired using standard protocols.11,12 The scans were anonymized, batched, and analysed (Circulation, Siemens for CT and CMRtools, Cardiovascular Imaging Solutions, London, UK) in blinded fashion by two experienced operators (for full details of CT- and CMR-imaging protocols see Supplementary material online).
Pulmonary exercise testing
Patients underwent exercise testing using a modified Bruce treadmill test performed by an independent team at Royal Brompton NHS Trust. Patients were monitored throughout with tests being terminated by physiological markers (ST changes, arrhythmias, or chest pain) or by patient request.
Statistical design and analysis
The study was powered to the primary endpoint of change in LVEF at 3 months within each treatment group as measured by advanced cardiac imaging. The sample size was calculated to detect an improvement in LVEF of 3.5% with a power of 90% and significance level of 5%, as demonstrated by a contemporary meta-analysis of previous cell therapy Phase I/II trials.13 The standard deviation for the advanced imaging LV measurements was estimated as 4%. This equated to 13 patients in each group, with the addition of 2 patients per group to account for loss, to total 15 per group.
Data were analysed using a modified intention-to-treat approach, meaning that patients who did not reach the primary and secondary endpoints were not included in all analyses. Baseline demographic and clinical variables were summarized for each group of the study. Continuous variables are presented as means ± SD and categorical variables are presented as percentages. 95% confidence intervals (CIs) are given. Within group comparisons were performed using the paired T test. Post hoc analyses between-group comparisons of all four groups were performed using a one-way analysis of variance and on this basis comparisons between individual groups, e.g. the between peripheral group comparison and between IC group comparison, were established using the Bonferroni correction method. Chi square test was used to make between-group comparisons of changes in NYHA class. P-values are two sided with a value of <0.05 considered to indicate statistical significance. All statistical analyses were performed using SPSS version 19 (IBM Corp. Armonk, NY, USA) and graphs produced using Graphpad Prism version 5.0 (GraphPad Software, San Diego, CA, USA).
Role of the funding source
No role of sponsor/funders in design or conduct of study.
Results
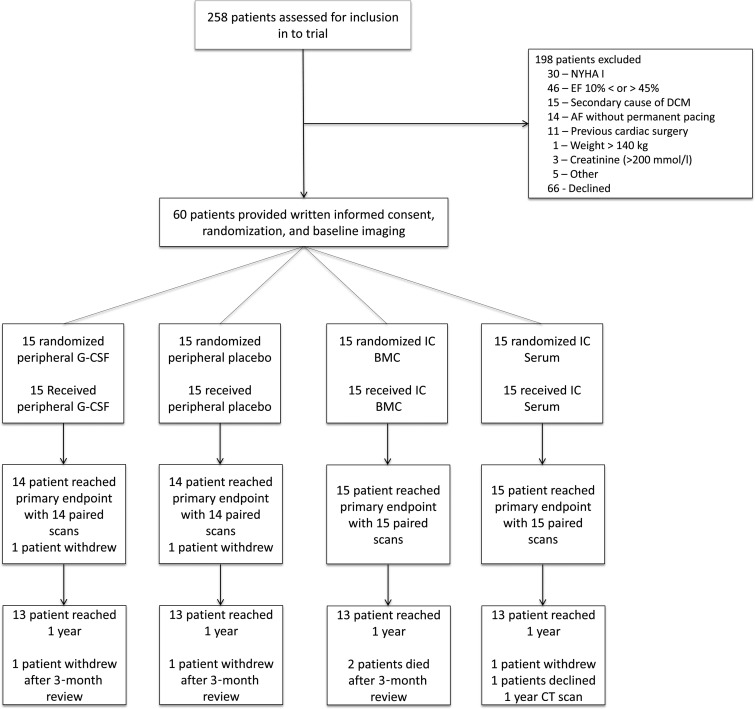
Between 6 July 2010 and 24 April 2012, 258 patients were screened from specialized heart failure clinics at the three referring centres. Of these patients, 132 were excluded for the following reasons: baseline NYHA classification <2 (n = 30), LVEF < 10% or >45% (n = 46) on referral centre echocardiogram, secondary cause of DCM (n = 15), atrial fibrillation without permanent pacing (n = 14), weight >140 kg (n = 1), creatinine >200 µmol/L (n = 3), and other (n = 16). Sixty-six patients declined participation. Of the 60 patients who were enrolled, DCM aetiology included: idiopathic (n = 49), post-myocarditis (n = 5), and familial disease (n = 6). Data are presented for the 60 patients who were randomized one of four groups: peripheral G-CSF (n = 15), peripheral placebo (n = 15), IC BMC (n = 15), and IC serum (n = 15) (Figure 1). A total of 58 patients reached the 3-month primary endpoint and 53 patients reached 1 year follow-up.
Figure 1.
Consort diagram. Flow chart of the study design summarizing flow of patients through the trial.
The mean age for the total population was 54.55 ± 11.19 years, and 70% were male. At baseline, all groups were similar with regard to age, sex, LVEF, plasma NT-proBNP concentration, or medical/ device management (Table 1). Changes in medications were minimal during the 3-month study period as inclusion criteria specified medical treatment had to be stabile for 6 months prior to treatment.
Table 1.
Baseline characteristics of the study population
| Peripheral placebo (n = 14) | Peripheral G-CSF (n = 14) | Intracoronary serum (n = 15) | Intracoronary BMC (n = 15) | P-value | |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 56.79 (9.84) | 54.57 (9.76) | 54.87 (10.86) | 57.67 (12.32) | 0.4395 |
| Sex (M/F) | 12/2 | 10/4 | 9/6 | 10/5 | 0.4307 |
| BMI (kg/m2), mean (SD) | 29.15 (4.48) | 29.19 (5.19) | 28.26 (9.10) | 27.23 (4.33) | 0.7338 |
| Hypertension, no. (%) | 2 (14.2%) | 1 (7.1%) | 1 (6.6%) | 2 (13.3%) | 0.8728 |
| Hypercholesterolaemia, no. (%) | 3 (21.4%) | 2 (14.2%) | 1 (6.6%) | 0 (0%) | 0.3081 |
| Diabetes mellitus, no. (%) | 2 (14.2%) | 1 (7.1%) | 1 (6.6%) | 2 (13.3%) | 0.8728 |
| Active smoker, no. (%) | 2 (14.2%) | 1 (7.1%) | 2 (13.3%) | 2 (13.3%) | 0.9279 |
| Family history of any heart disease, no. (%) | 2 (14.2%) | 1 (7.1%) | 2 (13.3%) | 2 (13.3%) | 0.3576 |
| Time from diagnosis to randomization (year), mean (SD) | 5.43 (0.98) | 7.6 (2.09) | 8.00 (1.61) | 4.9 (0.96) | 0.3132 |
| Medical therapy | |||||
| ACEi/ARB, no. (%) | 13 (92.9%) | 14 (100%) | 15 (100%) | 15 (100%) | 0.3997 |
| β-Blockers, no. (%) | 14 (100%) | 12(83.7%) | 13 (86.6%) | 13 (86.6%) | 0.3963 |
| Diuretics, no. (%) | 8 (57.1%) | 8 (57.1%) | 8 (53.3%) | 9 (59.9%) | 0.7349 |
| Aldosterone antagonists, no. (%) | 11 (78,6%) | 7 (50.0%) | 12 (79.9%) | 10 (66.6%) | 0.3356 |
| Digoxin, no. (%) | 2(14.2%) | 5 (35.7%) | 4 (26.6%) | 6 (39.9%) | 0.4282 |
| LVEF | |||||
| Baseline, mean (SD) | 29.75 (9.191) | 36.5 (13.26) | 41.70 (15.25) | 32.93 (16.46) | 0.1289 |
| Device therapy | |||||
| ICD, no. (%) | 3 (21.4%) | 5 (35.7%) | 4 (26.6%) | 4 (26.6%) | 0.8859 |
| Biventricular pacemaker, no. (%) | 1 (7.1%) | 0 (0%) | 2 (13.3%) | 2 (13.3%) | 0.5113 |
| CRT-D, no. (%) | 6 (42.9%) | 4 (28.6%) | 3 (19.9%) | 7 (46.6%) | 0.4077 |
BMI, body mass index; G-CSF, granulocyte colony-stimulating factor; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy defibrillator; LVEF, left ventricular ejection fraction.
Peripheral concentrations of CD34+ cells were significantly greater in patients who had G-CSF therapy (see Supplementary material online, Table S1). The average plasma CD34+ concentration increased from 3.94 ± 3.54/μL at baseline to 56.79 ± 45.13/μL after 6 days of G-CSF therapy; P = <0.0001. In the IC BMC group, the total mononuclear cell count in the injected cell product was 216.0 × 106 ± 221.8 with a mean percentage of viable cells of 98.2 ± 1.0 (see Supplementary material online, Table S2). The mean number of CD34+ cells in the cell product (as a measure of stem-like potential of the BMC population) was 4.91 × 106 ± 2.75. The mean granulocyte-macrophage colony forming unit capacity per 2 × 104 BMC (as a measure of functionality of the applied cells) was 7.42 ± 4.40.
Cells were injected into three coronary arteries in 10 patients and into the left anterior descending and circumflex arteries only in 5 patients. No cases of distal coronary artery occlusion, acute cardiac dysfunction, ventricular arrhythmia or significant CK or troponin T release occurred. One patient suffered a localized coronary dissection during infusion and was treated with coronary stenting but with no significant change in CK.
Left ventricular ejection fraction
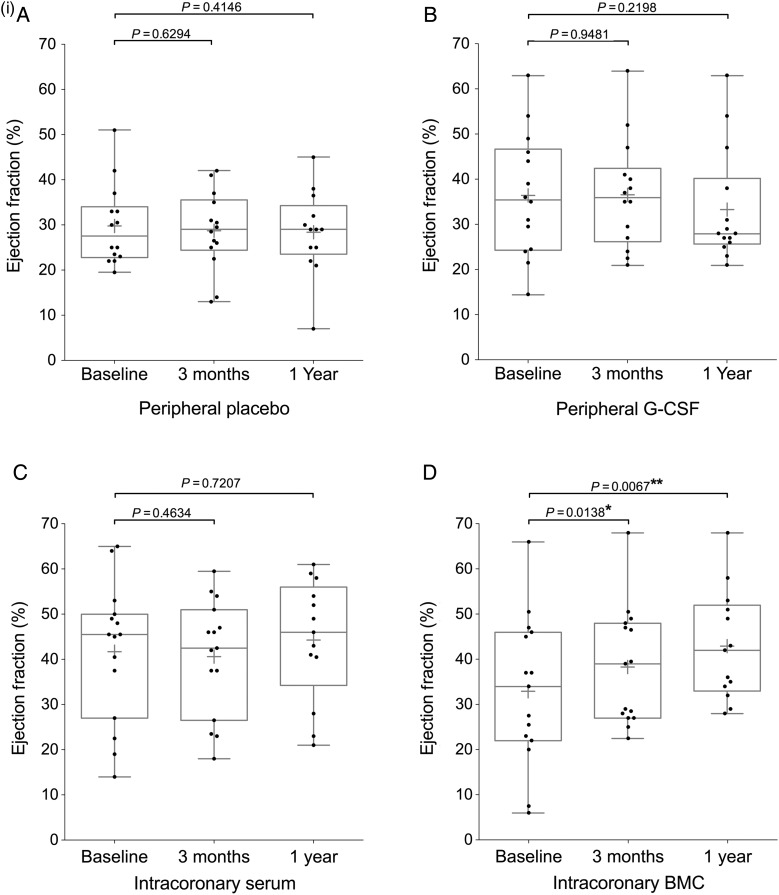
Twenty-two patients underwent CMR assessment of cardiac function and 38 CT assessment (see Supplementary material online, Table S3). Baseline measurements of LVEF did not differ significantly between the treatment groups (Table 1). Primary endpoint analysis revealed that the IC BMC group showed a 5.37% point increase (SD 7.39) in global LVEF from 32.93% ± 16.46 at baseline to 38.30% ± 12.97 at 3 months (P = 0.0138, n = 15). No significant change was seen in any of the other treatment groups between baseline and 3 months (peripheral G-CSF group mean difference: 0.14% ± 8.05; P = 0.9481, n = 14; peripheral placebo group mean difference: −1.07% ± 8.11; P = 0.6294, n = 14; serum group mean difference: −1.1 ± 5.65; P = 0.4634, n = 15) (Figure 2i).
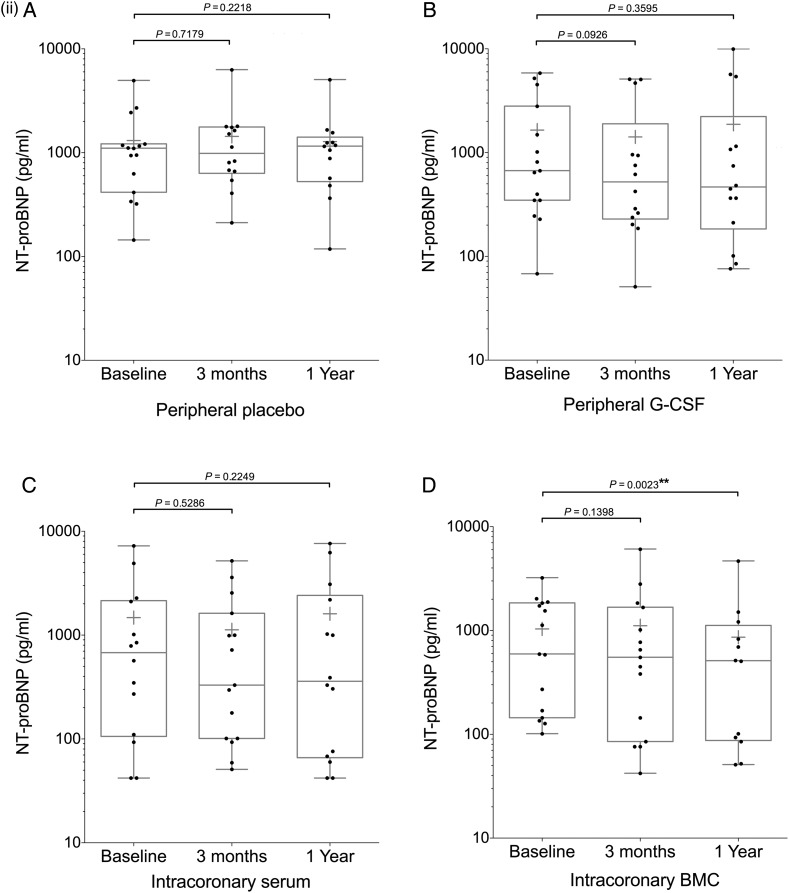
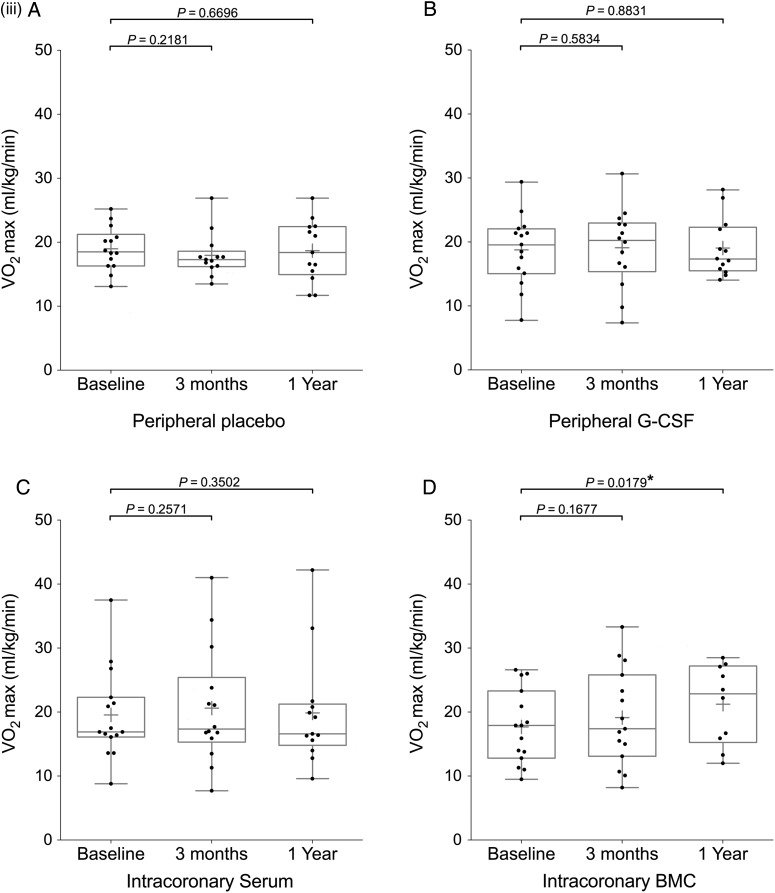
Figure 2.
Continued
Figure 2.
Continued
Figure 2.
Endpoint analysis of left ventricular ejection fraction, NT-proBNP, and VO2 max. Box and whisker plots (median and range, mean shown by +) including individual datapoints of primary and secondary endpoints of the REGENERATE-DCM trial measured at 3 months and 1 year. Endpoints: (i) left ventricular ejection fraction, (ii) NT-proBNP, (iii) VO2 max patient groups (A) peripheral placebo, (B) peripheral granulocyte colony-stimulating factor, (C) intracoronary serum infusion, and (D) intracoronary bone marrow-derived cell infusion.
Between-group analyses demonstrated the change in LVEF at 3 months was significantly greater in the IC BMC group compared with the IC serum group (absolute change in LVEF of 6.47%; P = 0.0119), and peripheral placebo group (absolute change in LVEF of 6.44%; P = 0.0430). The between IC BMC group and peripheral G-CSF group difference did not reach significance (absolute change in LVEF of 5.22%).
At 1 year the improvement in cardiac function was maintained in the IC BMC group with a 7.04% point increase (SD 7.77) in LVEF from baseline (42.92% ± 12.20 from 35.88% ± 15.45; P = 0.0067, n = 13). Again, at 1 year, the change in LVEF was significantly greater in the IC BMC group compared with all other treatment groups (P = 0.0109): IC serum group (P = 0.0420), peripheral placebo group (P = 0.0141), and peripheral G-CSF group (P = 0.0058). There was no significant difference in improvement of LVEF in patients who received BMC infusion in 3 or 2 coronary arteries. In the patients who showed improvement in LVEF at 1 year, there was a suggestion of an association between number of BMC injected and improvement in LVEF (r2 = 0.4848, P = 0.037, Supplementary material online, Figure S1).
No evidence of a difference was seen in LV end systolic volume (LVESV), LV end diastolic volume (LVEDV), stroke volume (SV), or myocardial mass (MM) over time in any of the treatment groups (see Supplementary material online, Table S3).
Plasma NT-proBNP concentration
NT-proBNP analysis was performed at baseline, 3 months and 1 year; results underwent logarithmic transformation due to non-normal distribution. There was significant decrease in NT-proBNP in the IC BMC group at 1 year (−136.0 pg/mL; 95% CI, −519.6–247.6; P = 0.0023) which was significantly greater than the change seen in the IC serum group (diff: 171.5 pg/mL; 95% CI, −200.6–543.6; P = 0.0420) (Figure 2ii; Supplementary material online, Table S4).
Exercise capacity
Fifty-six patients underwent pulmonary exercise testing at baseline and 3 months with 49 undergoing further assessment at 1 year. At 1 year, there was a significant improvement in VO2 peak in the IC BMC group (17.67 ± 5.76–21.23 ± 6.23 ml/kg/min; P = 0.0179) (Figure 2iii).
The IC BMC group showed a significant improvement in maximum exercise speed at 3 months (1.95 ± 0.71–2.55 ± 1.06 mph; P = 0.0192) and 1 year (1.95 ± 0.71–3.27 ± 1.06 mph; P = 0.0164) associated with an increase in exercise time at both time points (424.1 ± 183.2 s–504.0 ± 239.0 s; P = 0.0146 and 415.8 ± 183.7 s–578.1 ± 272.8 s, P = 0.0131 respectively) (see Supplementary material online, Table S5).
NYHA
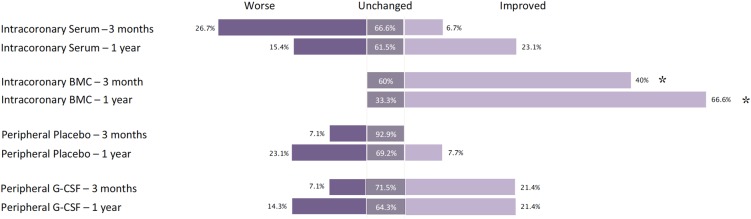
The percentage of patients who showed improvement in their NYHA classification was significantly higher in the IC BMC group (χ2 = 14.92, diff = 6; P = 0.02). In the IC BMC group with six (40%) patients showed improvement in NYHA class with no patients demonstrating a deterioration. In the IC serum group only one (6.7%) patient showed improvement, in the peripheral G-CSF group three (20%) patients showed improvement, and in the peripheral placebo group no patients showed improvement. At 1 year, this pattern continued with eight (66.7%) patients improving in the IC BMC group, three (23.0%) patients improving in the IC serum group, three (21.4%) patients improving in the peripheral G-CSF group, and one (8.3%) patients improving in the peripheral placebo group (χ2 = 12.61, diff = 6; P = 0.0497) (Figure 3).
Figure 3.
Change in NYHA. Bar chart showing symptomatic change as measured by change in NYHA classification per treatment group at 3 months and 1 year. Percentages reflect number of patients that have worsened, improved, or remain unchanged compared with baseline. (Note: unchanged is not represented by area). *Significance at P < 0.05.
Quality of life
Using the EQ5D index score and EQ5D visual analogue scale, there was no improvement in quality of life at 3 months in any of the treatment groups. At 1 year, the EQ5D index score showed evidence of improvement in quality of life in the peripheral G-CSF group only (0.462 ± 0.350–0.647 ± 0.236; P = 0.013) (see Supplementary material online, Table S6).
Using the KCCQ clinical summary score, there improvement in the patient's symptoms and social factors in the IC BMC group 3 months (54.64 ± 21.80–64.34 ± 25.83; P = 0.0028) and 1 year (63.67 ± 27.62; P = 0.0005 compared with baseline). The KCCQ overall summary score also showed an improvement at 1 year in both the IC BMC group (38.66 ± 20.96–56.27 ± 29.96; P = 0.0053 and the peripheral G-CSF group (50.80 ± 21.27–60.26 ± 21.16; P = 0.0438 (see Supplementary material online, Table S6).
Safety
There were no complications or adverse events associated with G-CSF therapy. Seven patients (15.6%) reported the common side effect of long bone pain during therapy. There was one report of MACE in each of the peripheral groups at 1 year, two reports of MACE in the IC BMC group and three reports of MACE in the IC serum group at 1 year. There were two deaths in the IC BMC group at 1 year due to causes unrelated to the trial: non-cardiac surgical procedure complication and bronchopneumonia (see Supplementary material online, Table S7).
Discussion
This is the first placebo-controlled trial of patients with DCM assessing the combination of cell and cytokine therapy to demonstrate a significant increase in cardiac function supported by improvements in symptoms, exercise physiology, and biochemical markers of heart failure. Granulocyte colony-stimulating factor alone did not have a beneficial effect on cardiac function supportive of recent meta-analysis data.14 The G-CSF/IC BMC patients demonstrated a 5.37% increase in LVEF at 3 months, which was maintained at 1 year. This LVEF improvement was associated with significant improvements in the clinical parameters of NYHA class, exercise capacity, quality of life decrease in NT-pro BNP at 1 year. The remaining treatment groups failed to show evidence of improvement in any of these endpoints at either 3 months or 1 year. These results therefore demonstrate the beneficial effects across multiple clinical and intermediate parameters of combined cell and cytokine therapy in a randomized control trial of patients diagnosed with DCM. Since the trial was designed to test whether BMC in addition to G-CSF provided added benefits it is not clear whether cell therapy alone would have had a similar effect.
Similar beneficial effects on cardiac function with BMC therapy have been shown in other early phase studies,15,16 with the most recent demonstrating improvements out to 5 years post therapy.9 However, no study has been performed with a randomized interventional control group blinded to the investigators. Previous studies have also used G-CSF alone but few have controlled for the possibility that this cytokine may have a direct effect on cardiac function as has been previously suggested.17 REGENERATE-DCM is the first trial designed with a separate interventional and cytokine only control group. Although the peripheral G-CSF group did not show an improvement in intermediate and clinical endpoints, there was an improvement in quality-of-life scores highlighting the need for rigorous study design involving appropriate control arms as previously suggested.7
The results presented here also support the hypothesis that the additional beneficial effects of IC BMC in combination with G-CSF compared with G-CSF alone are in part mediated by the local delivery of an enriched CD34+ cell population. Here CD34+ concentrations in the infused cell product are shown to be an order of magnitude above circulating CD34+ concentrations achieved by mobilization with G-CSF alone (491 CD34+/μL in cell product compared with 56.8 CD34+/μL in the circulation Supplementary material online, Tables S1 and S2).
The enrolled study population was typical of a DCM population with similar baseline characteristics and medical therapy to patients in other published trials.18,19 The biochemical markers indicating severity of heart failure were similar across all groups with a plasma NT-proBNP concentration >1000 pg/mL suggestive of significant left ventricular dysfunction. The patient population had high levels of optimal medical therapy and appropriate levels of ‘device therapy’ in all groups in keeping with current European guidelines.20
While patients were enrolled to the trial and randomized on the basis of referring echo criteria of LVEF <45%, their baseline LVEF measurements by MRI and CT were in some instances >45%, which is a recognized limitation of the study. Furthermore, although the baseline LVEF in the IC serum arm was increased compared with the other three groups, there was no significant between-group difference. It must also be noted here that although rate of MACE was not different between the treatment groups two deaths occurred within the IC BMC group which were unrelated to trial procedures and non-cardiac in aetiology. Although it is unusual that both events occurred within one group, a mortality rate of 2/60 may be reasonable in such a high-risk study population.
The results of the REGENERATE-DCM trial suggest a beneficial effect of combination G-CSF and BMC therapy; however, this is a Phase II trial powered around intermediate efficacy measures. The trial met its statistical endpoint criteria despite the small sample size, which was supported by relevant changes in the secondary endpoints. It is likely that small study size accounted for a failure to see significant changes in LVESV and LVEDV, that corresponded to improvement of LVEF, observations that have been linked in large meta-analyses.21 ***LVESV and LVEDV have a combined effect on LVEF therefore increasing sample size may have revealed significant changes. The study could not be completely blinded across all arms due to the invasive nature of the IC arm. Nonetheless, the investigators and patients were blinded within arm between active treatment and placebo and data analysers were blinded to all treatment groups. Although patients underwent either MRI or CT analysis since the scans were paired and the primary endpoint was based on within group measures any modality-related differences in the measurements of cardiac function would not account for the significant findings. Furthermore, the standard error of measurement of MRI and CT was similar.
Therefore, results of REGENERATE-DCM demonstrate improvement in a panel of measures of cardiac function accompanied by improvement in symptoms. In particular the change in plasma NT-proBNP at 1 year suggest that cell therapy may lead to a long-term outcome benefit in these patients and therefore warrants further investigation using the methodology described in this manuscript in a Phase III trial.
Conclusion
The IC infusion of autologous unfractionated BMC in combination with G-CSF therapy in patients with DCM appears to be safe and is associated with an improvement in LVEF 3 months after therapy, which is maintained at 1 year. These functional differences were accompanied by improvement in a panel of biochemical and symptom-related outcomes supporting a potential clinical benefit of this therapy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The trial was supported by unrestricted grants from the Heart Cells Foundation and Barts and the London Charity. Chugai Pharmaceutical donated supplies of G-CSF and pharmaceutical costs. Funding to pay the Open Access publication charges for this article was provided by the Barts Cardiovascular Biomedical Research Unit (CVBRU).
Conflict of interest: none declared.
Acknowledgements
We would like to thank the support of all the staff at the London Chest catheterization laboratories, The Heart Cell Foundation, Barts and the London Charity, and Chugai Pharma for their unrestricted support of this trial. M.R.C. and S.P. salaries were supported by the National Institute for Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital. The work at UCLH was supported by the UCLH Biomedical Research Centre. We also thank the data and safety and monitoring board members of Marcus Flather, Adam Timmis, Stephen Minger, and Gareth Ambler for their time and experience.
References
- 1.Hertz MI, Aurora P, Christie JD, Dobbels F, Edwards LB, Kirk R, Kucheryavaya AY, Rahmel AO, Rowe AW, Stehlik J, Taylor DO. Scientific Registry of the International Society for Heart and Lung Transplantation: introduction to the 2009 Annual Reports. J Heart Lung Transplant 2009;28:989–992. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. Am J Cardiol 1981;47:525–531. [DOI] [PubMed] [Google Scholar]
- 3.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med 1994;331:1564–1575. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010;375:752–762. [DOI] [PubMed] [Google Scholar]
- 5.Hill JM, Syed MA, Arai AE, Powell TM, Paul JD, Zalos G, Read EJ, Khuu HM, Leitman SF, Horne M, Csako G, Dunbar CE, Waclawiw MA, Cannon RP., III Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J Am Coll Cardiol 2005;46:1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2012;15:CD006536. [DOI] [PubMed] [Google Scholar]
- 7.Gho JM, Kummeling GJ, Koudstaal S, Jansen of Lorkeers SJ, Doevendans PA, Asselbergs FW, Chamuleau SA. Cell therapy, a novel remedy for dilated cardiomyopathy? A systematic review. J Card Fail 2013;19:494–502. [DOI] [PubMed] [Google Scholar]
- 8.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res 2015;116:1361–1377. [DOI] [PubMed] [Google Scholar]
- 9.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res 2013;112:165–173. [DOI] [PubMed] [Google Scholar]
- 10.Arnous S, Mozid A, Mathur A. The bone marrow derived adult stem cells for dilated cardiomyopathy (REGENERATE-DCM) trial: study design. Regen Med 2011;6:525–533. [DOI] [PubMed] [Google Scholar]
- 11.Kramer C, Barkhausen J, Flamm S, Kim R, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbara S, Arbab-Zadeh A, Callister T. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Latif A, Bolli R, Tleyjeh I. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167:989–997. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Latif A, Bolli R, Zuba-Surma EK, Tleyjeh IM, Hornung CA, Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomised controlled trials. Am Heart J 2008;156:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seth S, Bhargava B, Narang R, Ray R, Mohanty S, Gulati G, Kumar L, Airan B, Venugopal P, AIIMS Stem Cell Study Group. The ABCD (autologous bone marrow cells in dilated cardiomyopathy) trial a long-term follow-up study. J Am Coll Cardiol 2010;55:1643–1644. [DOI] [PubMed] [Google Scholar]
- 16.Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, Schächinger V, Tonn T, Martin H, Dimmeler S, Zeiher AM. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail 2009;2:417–423. [DOI] [PubMed] [Google Scholar]
- 17.Joseph J, Rimawi A, Mehta P, Cottler-Fox M, Sinha A, Singh BK, Pacheco R, Smith ES, III, Mehta JL. Safety and effectiveness of granulocyte-colony stimulating factor in mobilizing stem cells and improving cytokine profile in advanced chronic heart failure. Am J Cardiol 2006;97:681–684. [DOI] [PubMed] [Google Scholar]
- 18.Bansch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation 2002;105:1453–1458. [DOI] [PubMed] [Google Scholar]
- 19.Hoshikawa E, Matsumura Y, Kubo T, Okawa M, Yamasaki N, Kitaoka H, Furano T, Takata J, Doi YL. Effect of left ventricular reverse remodeling on long-term prognosis after therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and β blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2011;107:1065–1070. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 21.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circ 2012;126:551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]