Abstract
In utero hematopoietic cell transplantation (IUHCT) has been performed in Mucopolysaccharidosis Type VII (MPSVII) mice, but a lifelong engraftment of allogeneic donor cells has not been achieved. In this study, we sought to confirm a lifelong engraftment of allogeneic donor cells immunologically matched to the mother and to achieve partial rescue of phenotypes in the original MPSVII strain through IUHCT by intravenous injection. We performed in vitro fertilization in a MPSVII murine model and transferred affected embryos to ICR/B6-GFP surrogate mothers in cases where fetuses receiving IUHCT were all homozygous. Lineage-depleted cells from ICR/B6-GFP mice were injected intravenously at E14.5. Chimerism was confirmed by flow cytometry at 4 weeks after birth, and β-glucuronidase activity in serum and several phenotypes were assessed at 8 weeks of age or later. Donor cells in chimeric mice from ICR/B6-GFP mothers were detected at death, and were confirmed in several tissues including the brains of sacrificed chimeric mice. Although the serum enzyme activity of chimeric mice was extremely low, the engraftment rate of donor cells correlated with enzyme activity. Furthermore, improvement of bone structure and rescue of reproductive ability were confirmed in our limited preclinical study. We confirmed the lifelong engraftment of donor cells in an original immunocompetent MPSVII murine model using intravenous IUHCT with cells immunologically matched to the mother without myeloablation, and the improvement of several phenotypes.
Keywords: chimerism, immunotolerance, In utero hematopoietic cell transplantation, lysosomal storage disease
Introduction
In utero hematopoietic cell transplantation (IUHCT) is a promising approach to treat inherited diseases, but satisfactory engraftment has not yet be obtained, except in immunodeficiency diseases (Tiblad and Westgren 2008; Mattar et al. 2012). The cause of this problem has now been elucidated in animal models. In mice, engraftment is easily achievable in theory, but the actual engraftment of donor cells is competitively limited with host cells (Barker et al. 2003a). This competitive limitation was overcome by intravenous administration of a large amount of donor cells, as previously reported (Peranteau et al. 2007). At the same time, a lack of maternal immunization to donor alloantigen has been shown to be indispensable for a long-term engraftment (reviewed in ref. (Nijagal et al. 2012; Pearson and Flake 2013)). In this respect, maternal donor cells are beneficial to IUHCT (Nijagal et al. 2011; Parolini 2011) because they do not induce immunoreactions in the uterus, and maternal antibodies would not exclude engrafted donor cells in the fetuses.
The B6.C-H2bm1/ByBir-Gusmps mouse strain is an original MPSVII murine model of Sly disease (human MPSVII) (Sly et al. 1973), and lacks β-glucuronidase (GUSB) (Birkenmeier et al. 1982). Phenotypic characteristics of affected mice include, amongst others, decreased body length, short limbs, disproportionate dwarfism, short snout and premature death. The Gusmps mutation arose spontaneously and was found in the B6.C-H2bm1/By strain. As the allele, b haplotype mutation 1, was segregated from backcross strains based on responses to a skingraft rejection, donor cells from a background strain B6 are rejected by a MPSVII mouse and vice versa. MPSVII mice provide a good animal model of lysosomal storage diseases because the efficacy of treatments can be confirmed phenotypically (Vogler et al. 1990, 2005; Sands and Birkenmeier 1993; Casal and Wolfe 1998, 2000). In these mice, 1–5% of a normal enzyme expression level is sufficient for effective treatment (Wolfe et al. 1992; Donsante et al. 2007).
In utero hematopoietic cell transplantation has previously been performed in MPSVII mice, but a lifelong engraftment of allogeneic donor cells and a complete cure of the phenotype have not been achieved (Barker et al. 2001, 2003b; Casal 2001). For a successful long-term engraftment of allogeneic cells in IUHCT, the initial engraftment rate should be above the threshold of 1.8% (Durkin et al. 2008). Recent reports have shown that the threshold engraftment necessary for efficacious treatment in MPSVII mice could be accomplished through IUHCT by intravenous (IV) injection (Peranteau et al. 2007), and it has been suggested this is potentially achievable by using donor cells from the mother. The purpose of this study was to confirm a lifelong engraftment of allogeneic donor cells immunologically matched to the mother, and to improve the phenotypic aspects of the original MPSVII mouse strain using IUHCT by IV injection to administer a large amount of cells.
Materials and Methods
Mice
B6.C-H2bm1/ByBir-Gusmps/J (H2Kbml) mice were obtained from Charles River Laboratories Japan (Yokohama, Japan). This strain was maintained by breeding heterozygous mice. The genotype of each pup was identified by polymerase chain reaction (PCR) of DNA obtained via punch biopsy of the ear, as previously described (Wolfe and Sands 1996). Balb/c (H2Kd), C57BL/6 (H2Kb, referred to as B6) and ICR mice were purchased from either Sankyo Labo Service Corporation (Tokyo, Japan) or CLEA Japan (Tokyo, Japan). Mice expressing the EGFP gene were provided by Dr. Okabe (Osaka University). We backcrossed the green fluorescent protein (GFP) mice at least 10 times to the B6 strain and these were maintained (referred to as B6-GFP).
The F1 hybrid strain of ICR × N/B6-GFP (referred to as ICR/B6-GFP) was generated in our colony. Males were used as donors and females were used as surrogate mothers. ICR/B6-GFP surrogate mice received MPSVII embryos used for in vitro fertilization (IVF) and subsequently used for IUHCT in cases where fetuses receiving IUHCT were all homozygous. All animals were bred and maintained in the Laboratory Animal Facility of the National Center for Child Health and Development. Experimental protocols for this study were approved by the Institutional Animal Care and Use Committee at the National Center for Child Health and Development, and all animal experiments abided by the Declaration of Helsinki 1995 (as revised in Seoul 2008).
IVF and transfer of the two-cell-stage embryos (ET)
We performed IVF and ET according to procedures previously described (Kawano et al. 2014) with some modification. In brief, oocytes were collected from the oviductal ampulla region of superovulated female mice 14 to 16 h following a hCG injection, and were added to a 200 μL drop of HTF medium (Kyudo, Japan) covered with paraffin oil (Nacalai Tesque, Japan) equilibrated with 5% CO2 in air at 37°C. Sperm taken from the epididymis of 8- to 12-week-old male mice were induced to capacitate by incubating in HTF medium for 90 min in an atmosphere of 5% CO2 in air at 37°C before insemination. A final concentration of sperm 1 × 106 – 2.5 × 106 sperms/mL was added to the oocytes. At 6 h after the IVF, fertilized eggs with visible pronuclei were selected for ET, and the eggs were cultured overnight in a 50 μL drop of KSOM medium (Millipore UK, UK) until two-cell-stage embryos were developed. The two-cell-stage 7–13 embryos were then transferred to each oviduct of pseudopregnant surrogate mothers.
Donor bone marrow harvest
Whole bone marrow was harvested by flushing the tibias, femurs, and iliums with phosphate-buffered sucrose (PBS) solution. Low-density mononuclear cells were obtained as previously reported (Hayashi et al. 2002). Cells were washed twice and continued with lineage cell depletion for IUHCT. Lineage-depleted (Lin(-)) cells were obtained via magnetic cell sorting with the Lineage Cell Depletion Kit (Miltenyi Biotec, Germany) for the purpose of stem cell enrichment. More than 5 × enrichment magnification of Sca-1 positive cells was confirmed in viable Lin(-) cells.
In utero transplantation
We anesthetized pregnant mice with IV-injected isoflurane at E14.5. After a midline laparotomy was performed and the uterine horns were exposed, we injected Lin(-) cells adjusted to a concentration of 5 × 105 Lin(-) cells/5 μL into the fetal vitelline vein for each dam as previously described (Peranteau et al. 2007). Injections were limited to six fetuses or less in order to avoid unnecessary stress. IV injections were given under a dissecting microscope using pulled-glass micropipettes with a sharpened, beveled tip, and were confirmed by direct visualization. After all injections were completed, the uterus was returned to the abdominal cavity, and the abdominal incision was closed in two layers using absorbable 5-0 suture. Dams were kept warm during the procedure. Pups were delivered naturally, or by means of a cesarean section if pups were not born by the evening of the expected date of birth.
Analyses of donor chimerism
Chimerism levels of recipient mice were assessed by flow cytometry on a Cytomics FC500 (Beckman Coulter, USA) as the percentage of GFP-positive donor cells in mononuclear cell fraction and granulocyte fraction (Fig. 1). Peripheral blood was collected in heparinized capillary tubes by retroorbital sinus puncture at 4 and 8 weeks of age, and in some chimeric mice at every 8 weeks until 48 weeks of age. The blood was washed, and red blood cells were lysed using IOTest 3 Lysing Solution (Beckman Coulter, France). 7-AAD (Beckman Coulter, France) was used to exclude dead cells.
Figure 1.
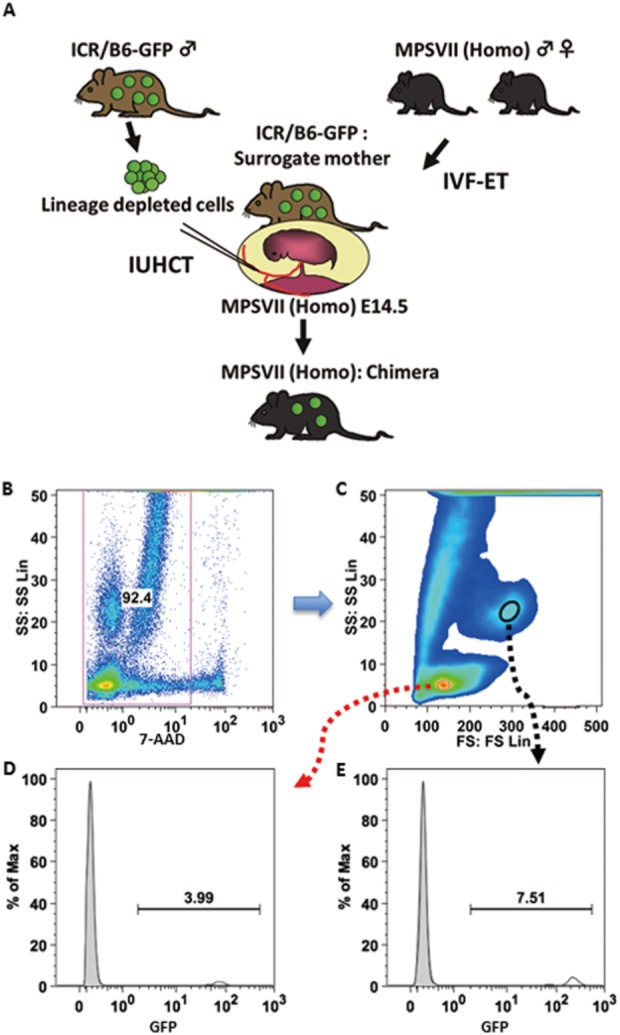
Chimeric mouse generated by In utero hematopoietic cell transplantation. Lineage-depleted cells from bone marrow of ICR/B6-GFP were administered into the vitelline veins of fetuses, which were developed from transferred homozygous embryos in the surrogate mother (A). Chimerism was confirmed by flow cytometry. Representative flow analysis of green fluorescent protein (GFP)-positive cells in a chimeric MPSVII mouse (B-E). (B) Gate for live cells. (C) Mononuclear cell fraction (red circle) and granulocyte fraction (black circle). (D, E) Donor engraftment rates in mononuclear cell fraction (D) and in granulocyte fraction (E) (see Table 1).
Biochemical analyses
Blood serum was collected by retroorbital sinus puncture from chimeric mice and age-matched controls. Serum GUSB activity was measured using 4-methylumbelliferyl-d-glucuronide (Sigma, USA) as substrate, described previously (Shapira et al. 1989) with some modification.
Microscopic analyses
Alive chimeric mice and control mice were sacrificed for perfusion with heparinized PBS and subsequently with 4% paraformaldehyde before preparation of the tissues. The tissues were equilibrated in a 10% sucrose solution (4°C, 30 min), and subsequently in a 15% sucrose solution (4°C, 30 min), then 20% (4°C, 1h) of 0.1 M PBS. They were frozen in O.C.T. compound (Sakura Finetek, USA), and then sectioned at 8 μm for histochemical and immunofluorescent staining. Histochemical analysis of GUSB activity in tissues where donor hematopoietic progenitor cells might engraft was performed using naphthol AS-BI β-D-glucuronide (Glycosynth, England) as a substrate described previously (Wolfe and Sands 1996). Sections were stained with Alcian blue (Muto Pure Chemicals, Japan) and counterstained with Nuclear fast red (Muto Pure Chemicals, Japan) to confirm specific pathological findings of affected tissues such as foamy cell appearance, although traditional fixation and staining dissolved stored mucopolysaccharide in expanded cells. For immunohistochemical analysis, frozen section slides were incubated with monoclonal antibodies of anti-GFP (ab290, Abcam plc, UK) overnight at 4°C, followed by horseradish peroxidase (HRP)-conjugated secondary antibody (Dako, Denmark). Staining was detected by diaminobenzidine and H2O2 on ice. Slides were counterstained with haematoxylin. For the immunofluorescence, DAPI was used for nuclear staining. Fluorescent images were acquired using BZ-9000 (Keyence, Japan).
Phenotype analyses
At 8 weeks of age, the body weight, femur length (between the edge of the proximal end of a greater trochanter and the midpoint between the distal ends of the lateral and medial condyles), and craniofacial phenotype of the mice were assessed. To quantify a craniofacial phenotype, we assessed the ratio of major nasal bone length and skull width measured at the widest points of the parietal bone (Fig. 4C). An in vivo microcomputed tomography (Latheta LCT-200; Hitachi Aloka Medical, Japan) was used to measure bone lengths. Mice were anesthetized by 2.0% isoflurane inhalation and scanned at a voxel size of 48 × 48 × 96 μm. Three-dimensional CT pictures of bones were reconstructed with VGStudio MAX2.0 software (Nihon Visual Science, Tokyo, Japan).
Figure 4.
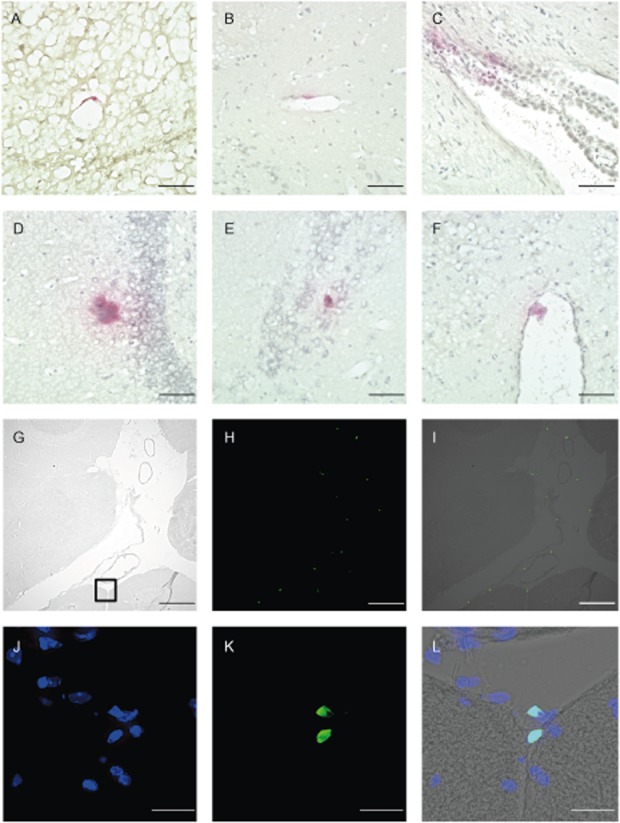
Donor cells detected in the brain of chimeric mice. Cells were stained for β-glucuronidase (GUSB) activity (A–F). (A–B) Cells at blood vessel wall, (C) Choroid plexus, (D–E) Cells in brain tissue, (F) Cells at pia mater in third ventricle, ×400. Scale bars: 50 μm (A–F). Detection of donor cells in leptomeningeal space (G–L). (G) Phase contrast microscopy, (H) green fluorescent protein (GFP) positive cells, (I) Merge, ×100. Scale bars: 200 μm (G–I). (J–L) Fluorescent microscopy. Enlarged view of the black square in (G). (J) Nuclear stained with 4′6′-diamidino-2-phenylindole dihydrochloride (DAPI), (K) GFP-positive cells at pia mater, (L) Merge. Original magnification, ×400 (J–L). Scale bars: 20μm (J-L).
To confirm whether the reproductive systems of chimeric MPSVII mice were recovered, they were mated with phenotypically normal chimeric mutant mice.
Statistical analyses
Statistical analyses were performed using Welch’s t-test for comparison of means with unequal variances or Mann–Whitney’s U-test for comparison of non-normally distributed data. The Bonferroni method was used for multiple comparisons. All tests were two-tailed, and a P-value of less than 0.05 was considered statistically significant.
Results
In vitro fertilization (IVF) with MPSVII homozygous embryos
The proportion of female MPSVII pups that survived weaning was 5.8% (n ½ 17/292) of newborn pups, which was about half of the theoretical rate (12.5%) as previously reported (Casal and Wolfe 1998). Furthermore, collecting eggs itself was a hard task. We collected an average of 9.3 eggs from an affected female, and 85% of them became two-cell-stage embryos. We first used Balb/c and B6-GFP mice for surrogate mothers, but too few embryos were developed at E13.5–E14.5. Next we used a larger ICR strain commonly used in murine IVF for surrogate mothers. Sixty-two embryos were transferred to ICR females, of which 29 (46.8%) fetuses were developed at the time of IUHCT. Similarly, of the 238 MPSVII embryos transferred to ICR/B6-GFP females, 50 (21.0%) fetuses were developed during the same period.
IUHCT of MPSVII fetuses
The established method of injection without lineage depletion to the vitelline vein (Peranteau et al. 2007) allows a larger amount of cells to be injected compared with intraperitoneal or intraliver injections (Casal 2001). In fact, intraperitoneal injections were performed but higher absolute levels of engraftment were achieved by delivering a larger cell dosage via the IV route; thus, this route and dose were used to assess the phenotypic correction of MPSVII mice by IUHCT.
We performed IUHCT of fetuses in ICR/B6-GFP dams with ICR/B6-GFP Lin(-) cells from male siblings. Vitelline veins in 10 out of 16 fetuses developed from 96 transferred embryos were thick enough for injection, and cells were completely administered by direct visualization. Ten pups survived weaning and donor cells were confirmed in 4 mice at 4 weeks of age (Fig. 1, Table 1). Chimerism in the surviving mice was confirmed by flow cytometry every 8 weeks after 8 weeks of age (data not shown).
Table 1.
Features of chimeric MPSVII mice injected intravenously
| Mouse name | GFP positive cells (%) | Body weight (g) | Femur length (mm) | S/NB ratio† | GUSB activity | Life span and other features | |||
|---|---|---|---|---|---|---|---|---|---|
| In mononuclear cell fraction | In granulocyte fraction | ||||||||
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | ||||||
| Male 1 | 0.20 | 0.11 | 2.53 | 1.79 | 21.1 | 12.42 | 1.60 | 0 | Sacrificed at 253 days‡ |
| Female 1 | 0.64 | 0.20 | 1.61 | 0.56 | 20.2 | 12.03 | 1.71 | 0.14 | 148 days§ |
| Male 2 | 2.17 | 1.54 | 10.4 | 8.58 | 27.4 | 13.72 | 1.78 | 1.57 | Sacrificed at 360 days |
| Female 2 | 4.12 | 3.5 | 14.96 | 16.75 | 23.5 | 13.71 | 1.49 | 1.59 | Sacrificed at 342 days |
S/NB ratio: ratio of the skull width to the nasal bone length (see Fig. 6C).
Mated with Female 2 and showed reproductive ability.
Mated with Male 1 and reproduced eight pups at first birth. Died after 2nd birth.
Detection of donor cells and GUSB activity
Serum GUSB activity was measured in chimeric mice in which donor cells were detected at 8 weeks after birth. As with homozygous mice, the enzyme activity was extremely low (Fig. 2), but as shown by regression analyses of chimeric mice the engraftment rate of donor cells seemed to be correlated with enzyme activity (R ½ 0.857) (Table 1).
Figure 2.
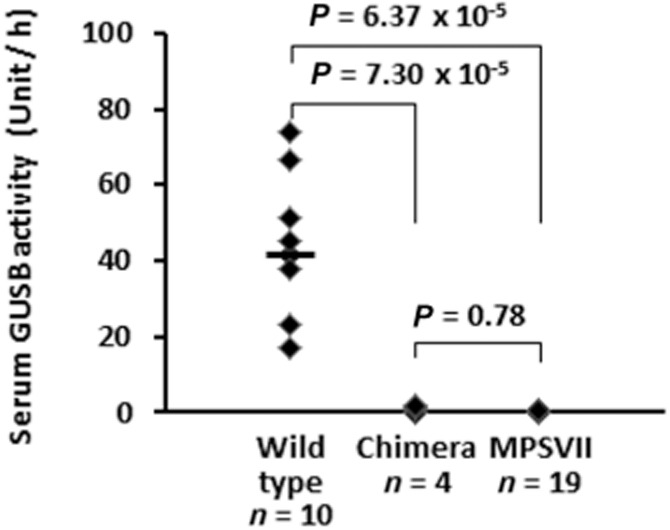
Comparison of serum β-glucuronidase (GUSB) activity. Chimeric mice in which donor cells were detected at 8 weeks after birth had little GUSB in serum. The horizontal line represents the median in wild-type mice (wild 41.7, chimera 0.86, and MPSVII 0.19, respectively). Welch’s t-test and the Bonferroni method were used for multiple comparisons.
Engraftment of donor cells was identified by fluorescent microscopy, immunohistochemistry was performed using an antibody to GFP (Supplementary Fig. S1), and histochemical analyses of GUSB activity was also performed. Donor cells were confirmed in several non-hematopoietic tissues including the brains of chimeric mice as well as in hematopoietic tissues, but not in affected mice (Fig. 3, 4, 5). More cells were confirmed in mice with a higher level of chimerism as previously reported (Hofling et al. 2003), and also pathological improvement was observed in the limited area close to the GUSB-positive cells (Fig. 5).
Figure 3.
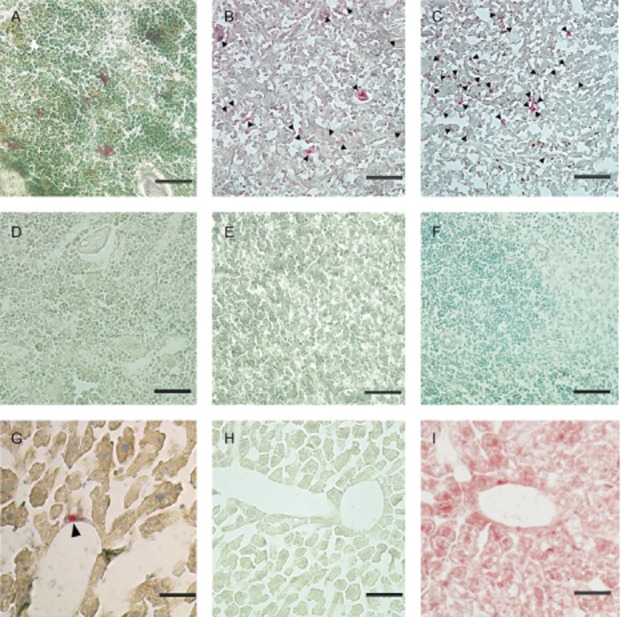
Histochemical analysis of β-glucuronidase (GUSB) activity. Engraftment of donor cells in chimeric mice after 250 days of age was confirmed by microscopic analyses. Frozen tissue sections were stained with Naphthol AS-BI β-D-glucuronide and counterstained with methyl green. Donor GUSB+ cells (red staining) are visible among the host GUSB-cells in several tissues (A-C, G). Black markers indicate red staining in (B), (C), and (G). (A) Bone marrow, (B) Thymus, (C) Spleen, (D) Liver, and negative control, respectively (D-F, H). (I) Positive control for liver. All cells are stained red. × 400 (A-E). Scale bars: 50μm.
Figure 5.
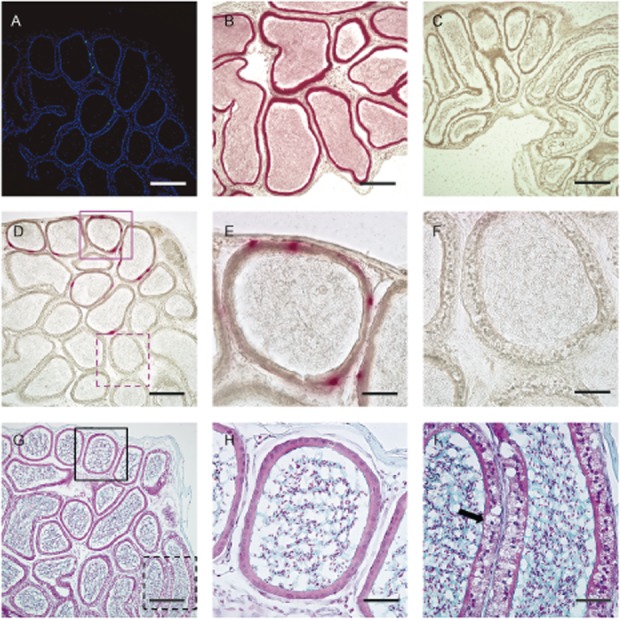
Representative staining of cross-correction by engrafted donor cells that produce β-glucuronidase (GUSB) in local areas. We analyzed frozen section slides of the epididymis sectioned close together, which were obtained from the chimeric mouse that had been rescued from infertility. (A) Fluorescent microscopy. Merge of green fluorescent protein (GFP) expressed by donor cells and nuclear, stained with 4′6′-diamidino-2-phenylindole dihydrochloride (DAPI). GFP-positive cells that had differentiated and engrafted in a limited part of the section could be seen. (B–F) Stain for GUSB activity, (B) Positive control, (C) Negative control for epididymis, ×100. Scale bars: 200 μm (A-D, G). (D) GUSB-positive cells are visible in a limited area, and the locations of some cells are the same as GFP-positive cells, shown in (A). (E–F) Enlarged views of the red-stained area (E) and the unstained area (F) from (D). (G–I) Staining with Alcian blue and counterstained with Nuclear fast red. (H–I) Enlarged views of the epididymis ducts (H, I) from (G) in a neighboring section of the red-stained area (E) and the unstained area (F). Normal appearance of epididymis ducts that contain GUSB-producing cells (E, H). Although traditional fixation and staining dissolves mucopolysaccharide in affected cells, a ballooning foamy cell appearance (arrow) can be seen in (I), ×400. Scale bars: 50μm (E–F, H–I).
Results of phenotypic analysis in chimeric MPSVII mice
Body weight and bone length are often used for phenotype assessment, and both parameters were examined in male and female chimeric mice and homozygous mice. Although the sample size was limited, the statistical difference tended to be significant (Table 2).
Table 2.
Body weight and femur length of male and female chimeric and control mice
| Plus-minus values are means ± SD | |||
|---|---|---|---|
| Body weight | Femur length | ||
| Male | Wt or hetero (n ½ 17) | 25.3 ± 2.5 | 14.8 ± 04 |
| Chimera (n ½ 2) | 21.1, 27.4 | 12.4, 13.7 | |
| Homo (n ½ 15) | 18.6 ± 3.6 | 12.0 ± 0.4 | |
| Female | Wt or hetero (n ½ 19) | 20.4 ± 0.8 | 14.4 ± 0.2 |
| Chimera (n ½ 2) | 20.2, 23.5 | 12.0, 13.7 | |
| Homo (n ½ 13) | 16.7 ± 1.7 | 11.0 ± 0.5 | |
Blind measurements of control mice were taken, and Mann–Whitney’s U-test was performed for statistical analyses between chimeric mice and homozygous mice. Statistical differences existed (P-value ½ 0.025–0.074).
A short snout is another characteristic phenotype of MPSVII mice, and we assessed this craniofacial phenotype by means of numeric conversion with a CT scan. The ratio of skull width to nasal bone length (S/NB ratio) was compared among wild-type/heterozygous mice, homozygous mice and chimeric mice (Fig. 6). A combined analysis of the craniofacial phenotype using both male and female mice was performed, as there was no statistically significant gender difference found for this phenotype in wild-type/heterozygous mice and homozygous mice (data are not shown). S/NB ratios between chimeric mice and wild-type/heterozygous mice were significantly different (Fig. 6E); however, the S/NB ratio of the most donor-engrafted mouse was the same as that of wild-type/heterozygous mice (Table 1). The fur of chimeric mice remained glossy and appeared normal.
Figure 6.
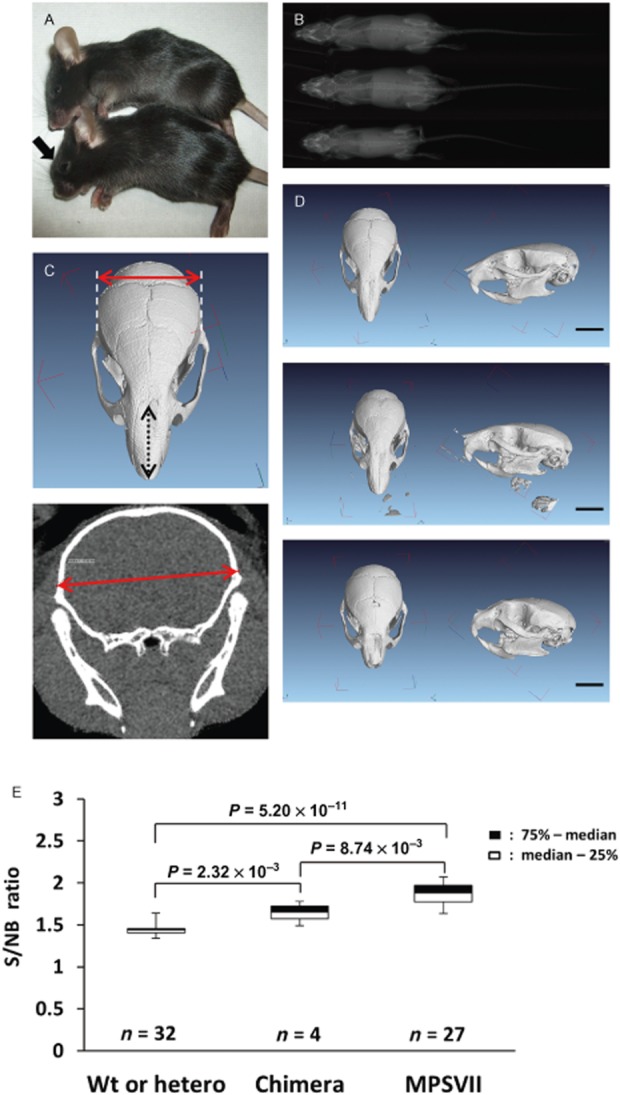
Analyses of craniofacial phenotype. (A) Phenotypically normal chimeric MPSVII mouse (top) and affected MPSVII mouse (bottom). Note the blunted face (arrow). (B) Radiograph of wild mouse (top), chimeric MPSVII mouse (middle) and affected MPSVII mouse (bottom) at 8 weeks of age. This chimeric mouse was as large as the wild mouse. (C) Major length of the nasal bone (black two-way arrow) and skull width at the widest points of the parietal bone (red two-way arrow). (D) Three-dimensional computed tomography (CT) scan of the skulls of a wild-type mouse (top row), a chimeric mouse (middle row) and an MPSVII mouse (bottom row) at 8 weeks after birth. Note the short nasal bone of the MPSVII mouse. Scale bars: 5 mm. (E) Box plot of the skull width to nasal bone length (S/NB) ratios at 8 weeks of age. Blind measurements of control mice were taken, and multiple comparisons were performed. Mann–Whitney’s U-test and subsequently the Bonferroni method were performed for statistical analyses.
Affected pairs cannot reproduce, because the male MPSVII mouse is sterile and the female mouse cannot nurse, although she occasionally gives birth to 1–2 pups (Beamer and Coleman 1982). We mated female chimeric mice with chimeric male MPSVII mice, and one of the females gave birth twice. Eight newborn pups from the first pregnancy were not nursed and died. All were confirmed to be homozygous by PCR (data are not shown). The mother died after the second labor at the age of day 148, and all pups were assumed to be cannibalized (Table 1). Three remaining chimeric mice were dissected after 36 weeks of age. As previously reported, the lifespan of MPSVII mice is 150–200 days (Beamer and Coleman 1982), and the average lifespan of male MPSVII mice in our institute was 164 ± 48 days (means ± SD, n ½ 41). Excluding cases of perinatal maternal death, chimeric mice lived longer (Table 1).
Discussion
The initial purpose of our research was to overcome the barriers to lifelong engraftment by confirming a long-term engraftment in an original MPSVII murine model, and also to observe the effects of treatment by using donor cells that were allogeneic to fetuses but congenic to mothers in intravenous IUHCT. However, we could not use congenic strains because of technical issues with IVF. ICR/B6-GFP mice generated from an outbred strain ICR and a congenic strain B6-GFP were used instead. Cells from the siblings were used for injections, so 50% of those cells were estimated to be matched to the mother, and at least, injected cells were haploidentical and less immunoreactive.
Although competitive barriers might be overcome by administration of a large number of stem cells in a murine model (Peranteau et al. 2007), immunological barriers have not yet been resolved. Recent studies have reported that the mechanism of immunological barriers was due to maternal responses (Rio et al. 2005; Merianos et al. 2009; Alonso-Ferrero et al. 2011; Nijagal et al. 2011). In addition to these reports, since the discovery that multi-transfused patients form less antibodies against non-inherited maternal antigens (NIMAs) (Claas et al. 1988), the mechanism of reduced immunoreactivity to maternal alloantigens has been elucidated (Mold et al. 2008, 2010; Lissauer et al. 2009; Dutta and Burlingham 2011; Nelson 2012). From this point of view, maternal cells would be advantageous as donor cells, and they are also favorable for IUHCT because immunoreactions do not occur in the mother as have been reported.
Our study confirmed the lifelong engraftment of allogeneic donor cells which contained maternally matched cells and partial rescue of phenotypes in the original MPSVII strain through IUHCT by IV injection (Fig. 6, Table 2). In our assessment of phenotypes, we expected that the S/NB ratio would be a good index for the evaluation of IUHCT therapeutic effects in addition to body weight and bone length, and that it could be used to evaluate craniofacial phenotypes, although the correlation between the S/NB ratio and engraftment rate was not proved by regression analyses among chimeric mice. Actually, some wild or heterozygous mice were in a state of poor growth with deformities of the mouth and died young (life-span means ± SD: 78.4 ± 35.3 days, n ½ 7) due to unknown causes and, given the situation, the S/NB ratio would have been useful to assess body weight and bone length.
It has been reported that 1–5% of normal levels of enzyme expression cause remarkable therapeutic effects in a MPSVII murine model (Wolfe et al. 1992; Donsante et al. 2007), while at least around 10% of normal GUSB activity is required for humans (Scriver et al. 1995). However, we observed that several phenotypes were improved and enzyme activity correlated with the engraftment rate; although we could detect little GUSB activity in serum (Fig. 3) as has been reported (Hofling et al. 2003). This fact was consistent with the report that donor cells could be detected in tissues even if seldom confirmed in blood (Barker et al. 2003a) because some effects may occur by engrafting donor cells in limited local areas. While it has been reported that enzyme activity increased in the plasma after BMT (Poorthuis et al. 1994), it could be speculated that at least a minimum amount of engrafted GUSB-positive cells would be needed for detection of the enzyme in plasma, judging from the fact that GUSB is absorbed quickly after intravenous administration (Vogler et al. 1993).
β-glucuronidase activity in each tissue where donor cells had been engrafted by bone marrow transplantation was relatively several fold lower than that of bone marrow (Hofling et al. 2003), and the distribution of GUSB-positive cells in affected mice that received syngeneic or xeno transplantations in myeloabrated or immunodeficient murine models were both similar (Hoogerbrugge et al. 1987; Birkenmeier et al. 1991; Sands et al. 1993; Soper et al. 1999; Hofling et al. 2003). Furthermore, the difference in reconstruction among tissues seemed to depend on the distribution level of bone marrow-derived tissue macrophages that were engrafted by transplantations such as Kupffer cells (Poorthuis et al. 1994; Hofling et al. 2003). Judging from this information, bone as well as other tissues would be better reconstructed where more bone marrow-derived cells were engrafted. This point was encouraging because GUSB activity and therapeutic effects would be ameliorated if excellent engraftment rates were achieved as previously reported. Otherwise, at least a delay of disease onset or improvement of symptoms would be expected if donor cells were engrafted, and this might be possible with a re-transplantation from the same donor.
Hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) differentiate into various cells and these cells migrate and engraft in several tissues (Prockop 1997; Brazelton et al. 2000; Lagasse et al. 2000; Mackenzie and Flake 2001; Troeger et al. 2010), where they show cross-correction effects. In fact, although low-density mononuclear cells from the bone marrow of mice might contain MSCs according to the manufacturer’s information, we observed the differentiation of a bone marrow-derived stem cell into a myoepithelial cell, and pathological improvement in the limited area close to the GUSB-positive cells at the epididymis of the mouse with rescued reproductive ability (Fig. 5, Supplementary Fig. S2). Moreover, monocytes, T cells and MSCs can pass through the brain blood barrier (BBB) (Casal and Wolfe 2000; Abkowitz et al. 2009; Matsushita et al. 2011; Lyck and Engelhardt 2012), while enzymes cannot pass the BBB. Bone marrow transplantation is clinically performed in some lysosomal storage diseases after birth and therapeutic effects based on these phenomena are expected, but the curative effect is limited because of the extent of disease progression by that time. Although it was reported that monocyte contribution to tissue macrophages varies from no contribution for microglias and Langerhans cells to complete monocyte origin for intestinal lamina propria macrophages (Sieweke and Allen 2013), we confirmed the presence of donor cells in the brain as reported in previous studies (Fig. 4) (Barker et al. 2001, 2003a).
Chimerism was achieved, but only a limited number of MPSVII fetuses were available for transplantations. This was due to the difficulty of collecting homozygous eggs from a small number of homozygous females, which sustained ovulation ability, and to the low development rate of fetuses after embryo transfer. Although several phenotypes were improved and GUSB activity in serum correlated with the engraftment rate, the sample size was limited for assessing the correlation between phenotype improvement and engraftment rate. Furthermore, evaluation of brain function is needed to assess the relationship between donor cell engraftment in the brain and definitive phenotype improvement.
The advent of iPS cells has dramatically changed stem cell therapy, and new technologies such as TALEN (transcription activator-like effector nuclease) and the CRISPR/Cas system (clustered regularly interspaced short palindromic repeats) are expected to greatly contribute to the field. However, when it comes to stem cell therapy In utero, the time limit until birth restricts the range of cell sources available. Stem cells from the bone marrow of the mother are acquired relatively easily, and they also circulate through the whole body with the ability to migrate into several organs. Our preliminary data confirmed the lifelong engraftment of donor cells that were immunologically matched to the mother, and the improvement of several phenotypes. Importantly, maternal stem cells are immunologically advantageous in stem cell transplantation In utero, and thus hold great potential as a cell source in future applications.
In conclusion, we could observe a lifelong engraftment of donor cells in an original MPSVII murine model by IUHCT using a massive dose of stem cells that were allogeneic for fetuses and at the same time immunologically matched to the mother. Several phenotypes were improved in our limited preclinical study. Further research using maternally matched cells in large animal models is needed to investigate the ideal cell source and quantity of stem cells, and the best method to occupy sufficient niches for a good engraftment rate.
Acknowledgments
This research was supported by grants from the Ministry of Education, Culture, Sport, Science, and Technology (MEXT) of Japan; the Ministry of Health, Labour and Welfare Sciences (MHLW), Japan; and the National Center for Child Health and Development, Japan. We would like to express our sincere thanks to M. Endo for essential advice and help in establishing the intravenous In utero transplantation technique in our laboratory; to H. Makino, M. Yamazaki-Inoue and T. Uyama for providing expert technical assistance; to M. Kosuga, T. Tanaka and H. Kimura for advice; H. Saito for encouragement; to Y. Kajiyama, Y. Suehiro, K. Saito, and K. Ito for their secretarial work, and to E. Barber for her in-house editorial support.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1 Detection of GFP-positive donor cells with immunohistochemistry. Cells were stained with anti-GFP antibody followed by horseradish peroxidase- conjugated secondary antibody. Staining of donor cells was detected by DAB reaction. (A–D) Representative tissue sections of the chimeric mouse. (A) Bone marrow, (B) Spleen, (C) Thymus, (D) Liver counterstained with hematoxylin. (E) Positive control for liver from a B6 GFP mouse. Note that the expression level of GFP varied between cells. (F) Negative control, ×400. Scale bars: 50 μm.
Fig. S2 Fluorescent immunostaining of the epididymis with anti-αSMA antibody followed by Alexa 546 conjugated secondary antibody. The expression of αSMA in the engrafted cells suggested that the donor cells differentiated to myoepithelial cells. (A) Merged yellow cells were located outside of the columnar epithelial cells. Donor cells (green), αSMA (red), Nucleus (blue), ×400. Scale bars: 50μm. (B) Enlarged view of a white square in (A). Original magnification, ×400. (C) Immunohistochemistry of epididymis tissue section stained with anti-αSMA antibody (Sigma, A2547) and counterstained with hematoxylin in a consecutive section. Alpha-SMA positive brown cells are located around the ducts. Scale bar: 50 μm.
References
- Abkowitz JL, Sabo KM, Yang Z, Vite CH, Shields LE, Haskins ME. In utero transplantation of monocytic cells in cats with alpha-mannosidosis. Transplantation. 2009;88:323–329. doi: 10.1097/TP.0b013e3181b0d264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Ferrero ME, Valeri A, Yanez R, et al. Immunoresponse against the transgene limits hematopoietic engraftment of mice transplanted In utero with virally transduced fetal liver. Gene Ther. 2011;18:469–478. doi: 10.1038/gt.2010.160. [DOI] [PubMed] [Google Scholar]
- Barker JE, Deveau S, Lessard M, Hamblen N, Vogler C, Levy B. In utero fetal liver cell transplantation without toxic irradiation alleviates lysosomal storage in mice with mucopolysaccharidosis type VII. Blood Cells Mol Dis. 2001;27:861–873. doi: 10.1006/bcmd.2001.0453. [DOI] [PubMed] [Google Scholar]
- Barker JE, Schuldt AJ, Lessard MD, Jude CD, Vogler CA, Soper BW. Donor cell replacement in mice transplanted In utero is limited by immune-independent mechanisms. Blood Cells Mol Dis. 2003a;31:291–297. doi: 10.1016/s1079-9796(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Barker JE, Schuldt AJT, Lessard ML, Jude CD, Vogler CA, Soper BW. Donor cell expansion is delayed following nonablative In utero transplantation to treat murine mucopolysaccharidosis type VII. Exp Hematol. 2003b;31:1112–1118. doi: 10.1016/s0301-472x(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Beamer WG, Coleman DL. Adipose storage deficiency (asd) Mouse Newslett. 1982;67:21. [Google Scholar]
- Birkenmeier EH, Davisson MT, Beamer WG, et al. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989;83:1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier EH, Barker JE, Vogler CA, et al. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991;78:3081–3092. [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Casal ML. In utero transplantation of fetal liver cells in the mucopolysaccharidosis type VII mouse results in low-level chimerism, but overexpression of beta-glucuronidase can delay onset of clinical signs. Blood. 2001;97:1625–1634. doi: 10.1182/blood.v97.6.1625. [DOI] [PubMed] [Google Scholar]
- Casal ML, Wolfe JH. Variant clinical course of mucopolysaccharidosis type VII in two groups of mice carrying the same mutation. Lab Invest. 1998;78:1575–1581. [PubMed] [Google Scholar]
- Casal ML, Wolfe JH. Mucopolysaccharidosis type VII in the developing mouse fetus. Pediatr Res. 2000;47:750–756. doi: 10.1203/00006450-200006000-00011. [DOI] [PubMed] [Google Scholar]
- Claas FH, Gijbels Y, den-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- Donsante A, Levy B, Vogler C, Sands MS. Clinical response to persistent, low-level beta-glucuronidase expression in the murine model of mucopolysaccharidosis type VII. J Inherit Metab Dis. 2007;30:227–238. doi: 10.1007/s10545-007-0483-4. [DOI] [PubMed] [Google Scholar]
- Durkin ET, Jones KA, Rajesh D, Shaaban AF. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 2008;112:5245–5253. doi: 10.1182/blood-2007-12-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Burlingham WJ. Microchimerism: tolerance vs. sensitization. Curr Opin Organ Transplant. 2011;16:359–365. doi: 10.1097/MOT.0b013e3283484b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Peranteau WH, Shaaban AF, Flake AW. Complete allogeneic hematopoietic chimerism achieved by a combined strategy of In utero hematopoietic stem cell transplantation and postnatal donor lymphocyte infusion. Blood. 2002;100:804–812. doi: 10.1182/blood-2002-01-0016. [DOI] [PubMed] [Google Scholar]
- Hofling AA, Vogler C, Creer MH, Sands MS. Engraftment of human CD34+ cells leads to widespread distribution of donor-derived cells and correction of tissue pathology in a novel murine xenotransplantation model of lysosomal storage disease. Blood. 2003;101:2054–2063. doi: 10.1182/blood-2002-08-2597. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge PM, Poorthuis BJ, Mulder AH, et al. Correction of lysosomal enzyme deficiency in various organs of beta-glucuronidase-deficient mice by allogeneic bone marrow transplantation. Transplantation. 1987;43:609–614. doi: 10.1097/00007890-198705000-00001. [DOI] [PubMed] [Google Scholar]
- Kawano N, Araki N, Yoshida K, et al. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci U S A. 2014;111:4145–4150. doi: 10.1073/pnas.1320715111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lissauer DM, Piper KP, Moss PA, Kilby MD. Fetal microchimerism: the cellular and immunological legacy of pregnancy. Expert Rev Mol Med. 2009;11:e33. doi: 10.1017/S1462399409001264. [DOI] [PubMed] [Google Scholar]
- Lyck R, Engelhardt B. Going against the tide – how encephalitogenic T cells breach the blood-brain barrier. J Vasc Res. 2012;49:497–509. doi: 10.1159/000341232. [DOI] [PubMed] [Google Scholar]
- Mackenzie TC, Flake AW. Multilineage differentiation of human MSC after In utero transplantation. Cytotherapy. 2001;3:403–405. doi: 10.1080/146532401753277571. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Kibayashi T, Katayama T, et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502:41–45. doi: 10.1016/j.neulet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Mattar CN, Biswas A, Choolani M, Chan JK. The case for intrauterine stem cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2012;26:683–695. doi: 10.1016/j.bpobgyn.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Merianos DJ, Tiblad E, Santore MT, et al. Maternal alloantibodies induce a postnatal immune response that limits engraftment following In utero hematopoietic cell transplantation in mice. J Clin Invest. 2009;119:2590–2600. doi: 10.1172/JCI38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells In utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol. 2012;33:421–427. doi: 10.1016/j.it.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijagal A, Wegorzewska M, Jarvis E, Le T, Tang Q, MacKenzie TC. Maternal T cells limit engraftment after In utero hematopoietic cell transplantation in mice. J Clin Invest. 2011;121:582–592. doi: 10.1172/JCI44907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijagal A, Flake AW, MacKenzie TC. In utero hematopoietic cell transplantation for the treatment of congenital anomalies. Clin Perinatol. 2012;39:301–310. doi: 10.1016/j.clp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Parolini O. In utero hematopoietic stem-cell transplantation–a match for mom. N Engl J Med. 2011;364:1174–1175. doi: 10.1056/NEJMcibr1100692. [DOI] [PubMed] [Google Scholar]
- Pearson EG, Flake AW. Stem cell and genetic therapies for the fetus. Semin Pediatr Surg. 2013;22:56–61. doi: 10.1053/j.sempedsurg.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Endo M, Adibe OO, Flake AW. Evidence for an immune barrier after In utero hematopoietic-cell transplantation. Blood. 2007;109:1331–1333. doi: 10.1182/blood-2006-04-018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis BJ, Romme AE, Willemsen R, Wagemaker G. Bone marrow transplantation has a significant effect on enzyme levels and storage of glycosaminoglycans in tissues and in isolated hepatocytes of mucopolysaccharidosis type VII mice. Pediatr Res. 1994;36:187–193. doi: 10.1203/00006450-199408000-00009. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rio P, Martinez-Palacio J, Ramirez A, Bueren JA, Segovia JC. Efficient engraftment of In utero transplanted mice with retrovirally transduced hematopoietic stem cells. Gene Ther. 2005;12:358–363. doi: 10.1038/sj.gt.3302419. [DOI] [PubMed] [Google Scholar]
- Sands MS, Birkenmeier EH. A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci U S A. 1993;90:6567–6571. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands MS, Barker JE, Vogler C, et al. Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab Invest. 1993;68:676–686. [PubMed] [Google Scholar]
- Scriver CR, Beaudet AL, Sly WS, Valle D. In metabolic basis of inherited disease. 7th edn. New York, NY: McGraw-Hill; 1995. [Google Scholar]
- Shapira E, Blitzer MG, Miller J, Africk DK. Biochemical genetics: a laboratory manual. New York, Oxford: Oxford University Press; 1989. [Google Scholar]
- Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Soper BW, Duffy TM, Vogler CA, Barker JE. A genetically myeloablated MPS VII model detects the expansion and curative properties of as few as 100 enriched murine stem cells. Exp Hematol. 1999;27:1691–1704. doi: 10.1016/s0301-472x(99)00098-3. [DOI] [PubMed] [Google Scholar]
- Tiblad E, Westgren M. Fetal stem-cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2008;22:189–201. doi: 10.1016/j.bpobgyn.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Troeger C, Perahud I, Moser S, Holzgreve W. Transplacental traffic after In utero mesenchymal stem cell transplantation. Stem Cells Dev. 2010;19:1385–1392. doi: 10.1089/scd.2009.0434. [DOI] [PubMed] [Google Scholar]
- Vogler C, Birkenmeier EH, Sly WS, et al. A murine model of mucopolysaccharidosis VII. Gross and microscopic findings in beta-glucuronidase-deficient mice. Am J Pathol. 1990;136:207–217. [PMC free article] [PubMed] [Google Scholar]
- Vogler C, Sands M, Higgins A, et al. Enzyme replacement with recombinant beta-glucuronidase in the newborn mucopolysaccharidosis type VII mouse. Pediatr Res. 1993;34:837–840. doi: 10.1203/00006450-199312000-00028. [DOI] [PubMed] [Google Scholar]
- Vogler C, Levy B, Galvin N, Lessard M, Soper B, Barker J. Early onset of lysosomal storage disease in a murine model of mucopolysaccharidosis type VII: undegraded substrate accumulates in many tissues in the fetus and very young MPS VII mouse. Pediatr Dev Pathol. 2005;8:453–462. doi: 10.1007/s10024-005-0025-8. [DOI] [PubMed] [Google Scholar]
- Wolfe JH, Sands MS. Murine mucopolysaccharidosis type VII: a model system for somatic gene therapy of the central nervous system. In: Lowenstein PR, Enquist LW, editors. Gene transfer into neurones, towards gene therapy of neurological disorders. Essex: Wiley; 1996. pp. 263–274. In:, editors. [Google Scholar]
- Wolfe JH, Sands MS, Barker JE, et al. Reversal of pathology in murine mucopolysaccharidosis type VII by somatic cell gene transfer. Nature. 1992;360:749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Detection of GFP-positive donor cells with immunohistochemistry. Cells were stained with anti-GFP antibody followed by horseradish peroxidase- conjugated secondary antibody. Staining of donor cells was detected by DAB reaction. (A–D) Representative tissue sections of the chimeric mouse. (A) Bone marrow, (B) Spleen, (C) Thymus, (D) Liver counterstained with hematoxylin. (E) Positive control for liver from a B6 GFP mouse. Note that the expression level of GFP varied between cells. (F) Negative control, ×400. Scale bars: 50 μm.
Fig. S2 Fluorescent immunostaining of the epididymis with anti-αSMA antibody followed by Alexa 546 conjugated secondary antibody. The expression of αSMA in the engrafted cells suggested that the donor cells differentiated to myoepithelial cells. (A) Merged yellow cells were located outside of the columnar epithelial cells. Donor cells (green), αSMA (red), Nucleus (blue), ×400. Scale bars: 50μm. (B) Enlarged view of a white square in (A). Original magnification, ×400. (C) Immunohistochemistry of epididymis tissue section stained with anti-αSMA antibody (Sigma, A2547) and counterstained with hematoxylin in a consecutive section. Alpha-SMA positive brown cells are located around the ducts. Scale bar: 50 μm.


