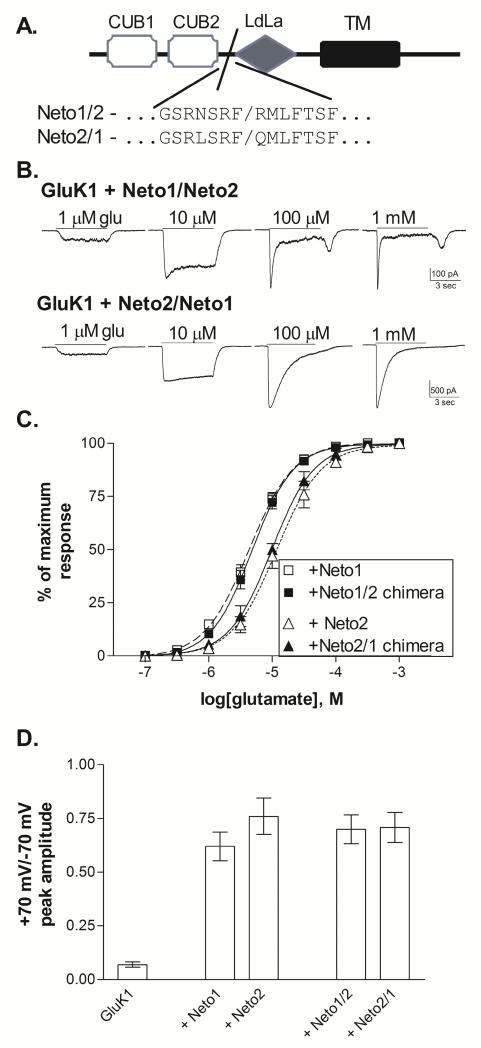
Figure 5. Functional effects of Neto1/Neto2 chimeric subunits.
A. Schematic representation of Neto subunit structure. The extracellular N-terminal sequence contains two CUB domains and one region with homology to the LDL-receptor class A repeat (LDLa) (Stöhr et al., 2002). The single transmembrane domain (TM) is followed by an intracellular C-terminal domain. The chimeric splice site is located at the end of the second CUB domain, prior to the LDLa domain. Neto1/2 chimeras contain Neto1 structure from the N-terminal domain up to the splice site, and Neto2 structure beyond the splice site while Neto 2/1 chimeras contain the reverse.
B. Representative whole-cell currents from transiently transfected HEK-293T cells. Glutamate was applied for 5 sec (solid line) at the concentration indicated to cells voltage-clamped at −70 mV.
C. Glutamate concentration-response relationships were constructed by normalizing the current amplitude to the peak response to a saturating concentration for each cell. Symbols represent mean±SEM and averaged data were fit with a sigmoidal function (lines). GluK1+Neto1 and GluK1+Neto2 data is repeated from Figure 1. Glutamate EC50s from the fits shown to the averaged data were 4.8 μM (Neto1/2, n=9) and 9.8 μM (Neto2/1, n=4).
D. Peak current amplitudes were measured in response to 5 sec. applications of 100 μM glutamate at holding potentials of +70 mV and −70 mV. The reversal potential was near 0 mV for all subunit combinations. Bars represent mean ± SEM (n=5 for all combinations). All Neto-containing receptors were significantly different (p≤0.001) from GluK1 without Neto, but were not different from one another (p>0.5).