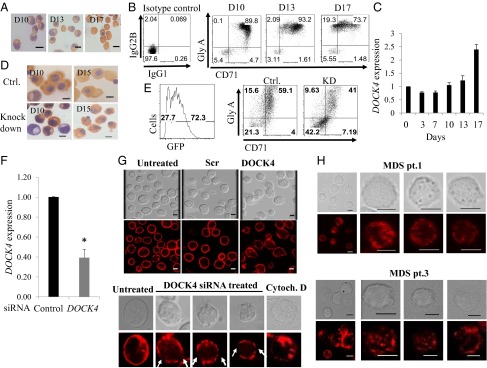
Fig. 3.
DOCK4 knockdown in primary human erythroblasts disrupts F-actin skeleton. (A) Photomicrographs depicting differentiating human primary erythroblasts on various days stained with benzidine and hematoxylin. D10, day 10 polychromatic erythroblasts; D13, day 13 orthochromatic erythroblasts; D17, day 17 reticulocytes. (B) Flow cytometry analysis of differentiating human primary erythroid progenitors for transferrin receptor (CD71) and Glycophorin A, showing gradual acquisition of surface markers that signify the erythroid program. (C) Quantitative RT-PCR (qRT-PCR) showing relative expression levels of DOCK4 transcripts during prelineage commitment (day 0) and postcommitted terminal differentiation into reticulocytes (P < 0.05, Student’s t test; n = 3). (D) Photomicrographs of differentiating human erythroblasts on various days stained with benzidine and hematoxylin following DOCK4 knockdown with shRNA against DOCK4 depicting multinucleated cells. Ctrl., control. (E) Flow cytometry analysis depicting the efficiency of DOCK4 knockdown (KD) (Left) and impeded differentiation as measured by the levels of glycophorin A (GlyA)/CD71 levels (Right). (F) qRT-PCR showing the extent of DOCK4 knockdown by siRNA on day 8 erythroblasts (*P < 0.05; Student’s t test). (G, Top) Immunofluorescence microscopy analysis showing disrupted actin filaments on day 8 erythroblasts after DOCK4 knockdown. Photomicrographs also depict cells that were treated with scrambled (Scr) or left untreated, with siRNAs along with corresponding differential interference contrast (DIC) images. (G, Bottom) Individual cells at high magnification showing gaps in the F-actin skeleton in a sample treated with DOCK4-specific siRNA and a sample treated with the actin-disrupting agent cytochalasin D (Cytoch. D) as a positive control. A cell from an untreated sample was used as the negative control. (Scale bars, 5 μm.) (H) DIC and immunofluorescence micrographs of day 10 (orthochromatic erythroblasts) derived from MDS patients showing disrupted F-actin skeleton. (Scale bars, 5 μm.)