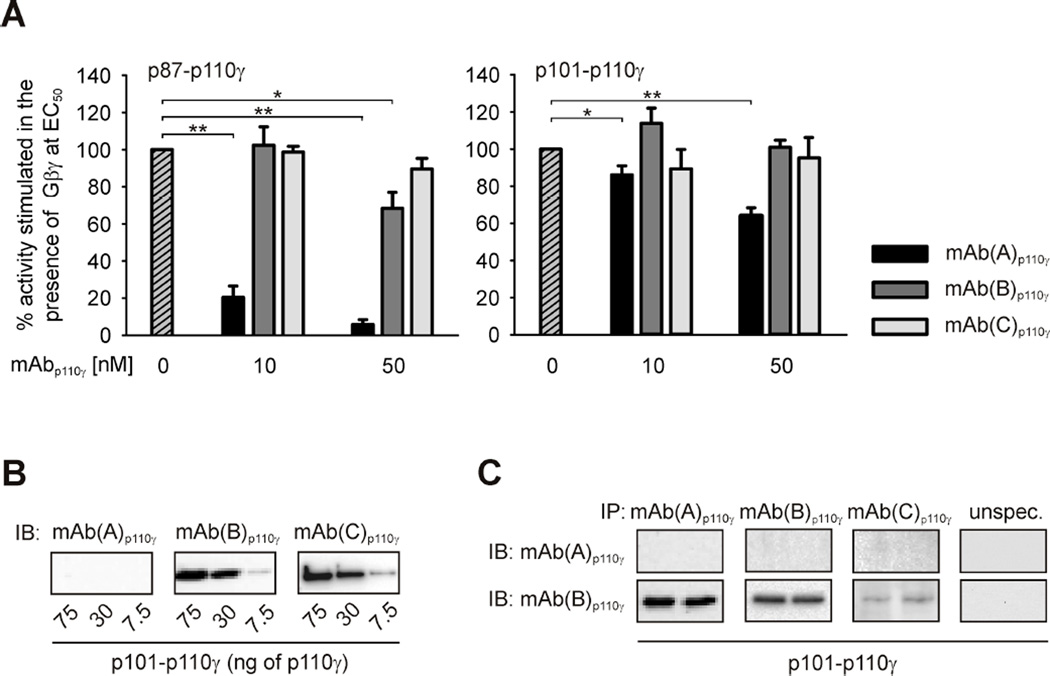
Figure 4. Different impact of monoclonal anti-p110γ antibodies on enzymatic activity of heterodimeric PI3Kγ variants.
(A) Monoclonal anti-p110γ antibodies, mAb(A)p110γ, mAb(B)p110γ and mAb(C)p110γ, were tested for their ability to affect Gβ1γ2-induced lipid kinase activity of purified recombinant p87-p110γ and p101-p110γ. PI3Kγ variants (1.5 nM) were stimulated by Gβ1γ2 at EC50 values, i.e. 300 nM for p87-p110γ and 30 nM for p101-p110γ. The data shown here are mean values ± S.E. (n = 3). (B) mAb(A)p110γ does not interact with catalytic p110γ subunit of denatured p101-p110γ in immunoblots. Heterodimeric enzyme was expressed in and purified from Sf9 cells. Different amounts of the recombinant protein were subjected to SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) using monoclonal anti-p110γ antibodies, mAb(A)p110γ, mAb(B)p110γ or mAb(C)p110γ. (C) mAb(A)p110γ binds catalytic subunit of intact p101-p110γ. Purified recombinant p101-p110γ was subjected to immunoprecipitation (IP) using mAb(A)p110γ, mAb(B)p110γ, mAb(C)p110γ or p110γ-unspecific antibody as described in the Experimental section. Duplicates of immunoprecipitates were separated by SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) with mAb(A)p110γ or mAb(B)p110γ.