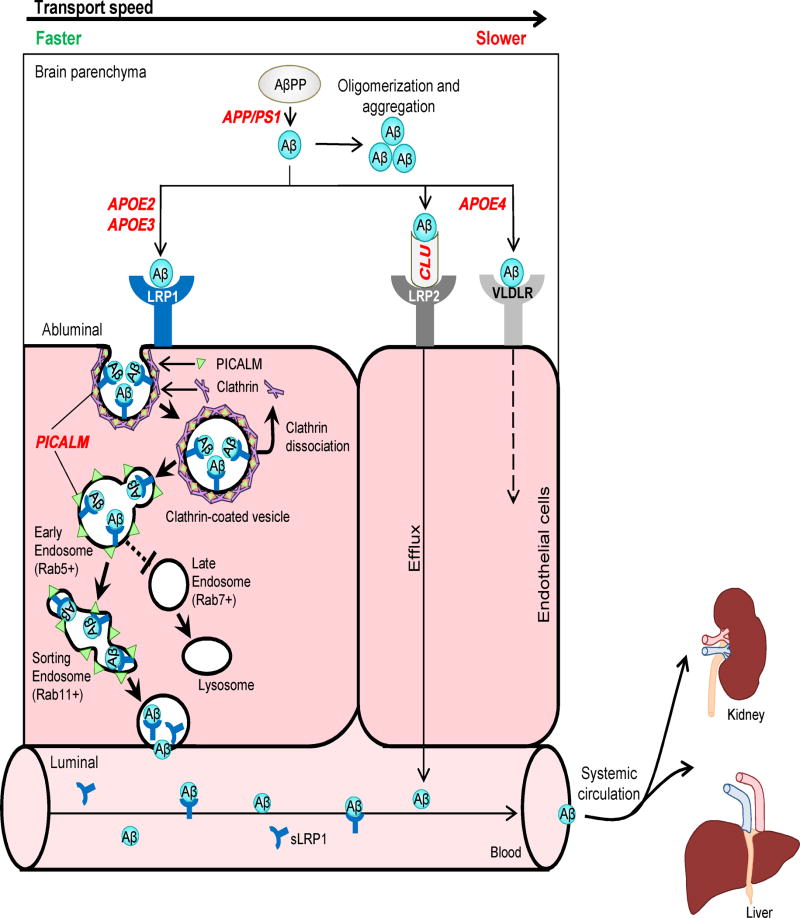
Figure 6. Alzheimer’s amyloid β-peptide clearance across the blood-brain barrier.
Transvascular Aβ clearance. LRP1 binds Aβ at the abluminal side of endothelium, which recruits PICALM, resulting in PICALM/clathrin-dependent endocytosis of LRP1-Aβ complexes. Next, PICALM guides the trafficking of Aβ-LRP1 endocytic vesicles to Rab5+ early endosomes and then to Rab11+ sorting endosomes for exo-cytosis at the luminal side of the BBB, resulting in Aβ transcytosis. PICALM guides Aβ away from Rab7+ late endosomes and lysosomes. Apolipoprotein J (apoJ; CLU) facilitates Aβ42 clearance across the BBB via LRP2.
Systemic Aβ clearance. Aβ binds to soluble LRP1 (sLRP1) in plasma. Circulating sLRP1-Aβ complexes are transported to liver and kidney for elimination from the body.
Genetic risk factors. APOE2 and APOE3 carry lower risk for AD compared to APOE4. CLU variants influence risk for sporadic AD, but their effects on Aβ clearance are presently unknown. Some protective PICALM variants lead to increased PICALM expression and enhanced Aβ clearance across the BBB. PSEN1 mutations causing early autosomal dominant AD lead to increased production of Aβ, particularly Aβ42, which increases Aβ load for clearance across the BBB.