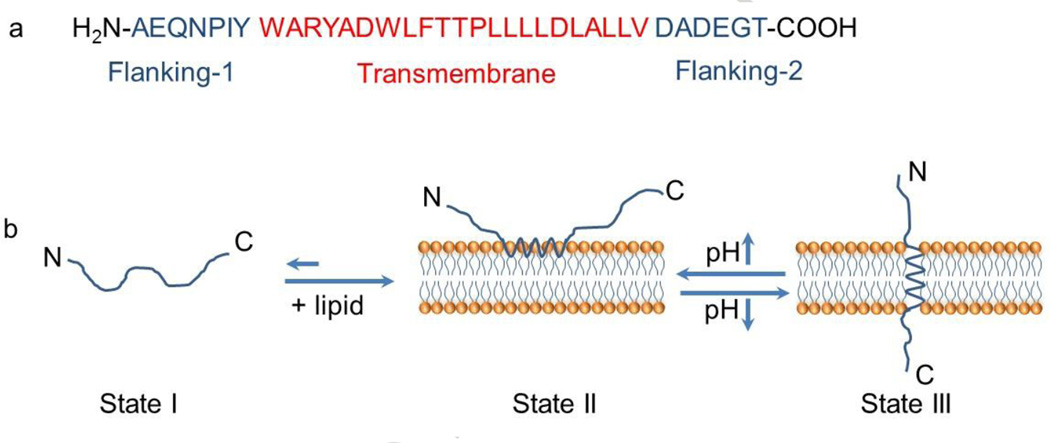
Figure 4.
(a) The amino acid sequence of a pH-responsive insertion peptide. (b) A schematic representation of the peptide in solution and interacting with a lipid bilayer at neutral (pH 7.0) and acidic pH (below pH 6.0). State I refers to the peptide in solution at normal and basic pHs. Upon addition of lipid, the unstructured peptide is adsorbed on the membrane surface (State II). The drop of pH leads to the protonation of Asp/Glu residues, increasing peptide hydrophobicity, and resulting in the insertion and formation of a transmembrane α-helix (State III). Adapted from Ref [59] with permission from the National Academy of Sciences.