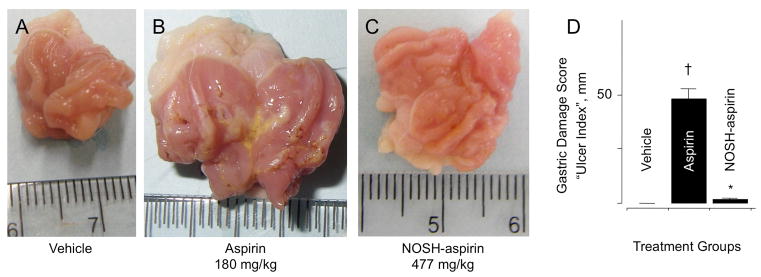
Figure 2.
NOSH-aspirin does not cause gastric damage. Aspirin and NOSH-aspirin were administered orally at equimolar doses (1 mmol/kg) and effects on the stomach were evaluated 6 hrs post-administration as indicated in Section 2.2. Panel A, shows the stomach of a vehicle-treated rat; Panel B, stomach of an aspirin-treated rat showing ulceration and bleeding; Panel C, stomach of a NOSH-aspirin-treated rat which is devoid of ulcers. Panel D, gastric damage due to aspirin, UI = 48 ± 4 mm (†P < 0.01 compared to vehicle), whereas NOSH-aspirin was gastric damage-sparing, UI = 2 ± 0.5 mm (*P < 0.01 compared to aspirin).