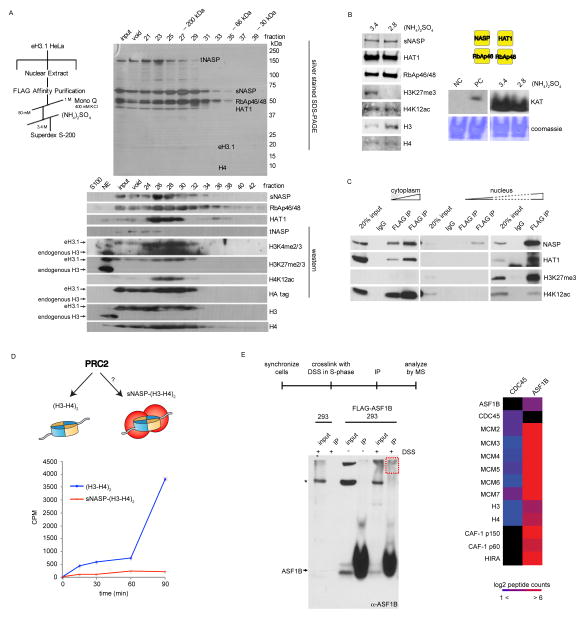
Figure 4.
Molecular functions of nuclear sNASP and ASF1B. (A) Nuclear sNASP co-elutes with HAT1 and evicted histones. (B) Both sNASP-HAT1 complexes (with or without evicted histones) are enzymatically active. Left panel: Western analysis of peak eH3.1 fractions containing sNASP with evicted or new histones (precipitated at 3.4 and 2.8 M ammonium sulfate, respectively). Right panel: Acetyltransferase activity towards histones demonstrated by autoradiography of acetyl-[3H] incorporation. (C) Nuclear, but not cytosolic sNASP co-precipitates histones with marks characteristic of eu- and hetero-chromatin. (D) In vitro PRC2 methyltransferase assay. sNASP-bound histones are poor substrates for the PRC2 complex compared to free soluble (H3-H4)2 tetramers. (E) In vivo crosslinking of replicating 293 cells (left panel), and mass spectrometry analysis of crosslinked, immunoprecipitated, ASF1B.