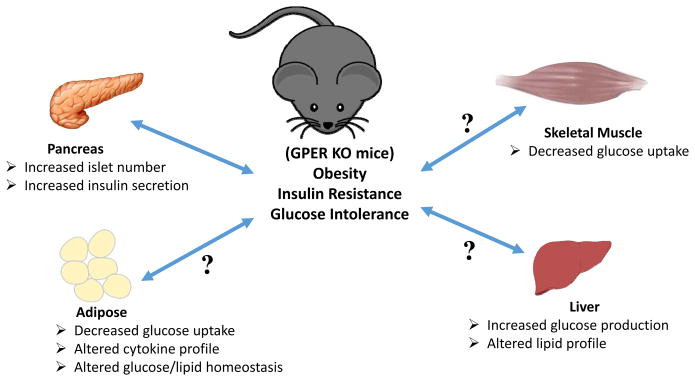
Figure 1. Schematic representation of the possible metabolic dysfunctions leading to or resulting from obesity in GPER-deficient mice.
Increased adiposity results in insulin resistance with ensuing glucose intolerance. Obesity may also result in multiple defects in metabolically active tissues such as the pancreas, adipose, liver and muscle. In the pancreas, insulin resistance leads to increased insulin secretion and a compensatory increase in the number of islets, resulting in hyperinsulinemia. In adipose and muscle, insulin resistance may lead to decreased insulin-stimulated glucose uptake, which along with concomitantly increasing hepatic glucose production, leads to hyperglycemia. In addition, altered lipid homeostasis in the adipose and liver lead to hyperlipidemia. Together with hypertension, these pathophysiological metabolic effects (abdominal obesity, elevated fasting plasma glucose, high serum triglycerides, and low high-density cholesterol levels) comprise the condition referred to as metabolic syndrome. Whereas GPER is known to stimulate insulin secretion and survival of pancreatic beta cells, little is known regarding the role(s) of GPER in other insulin-responsive peripheral organs/tissues (as indicated by question marks (?)).