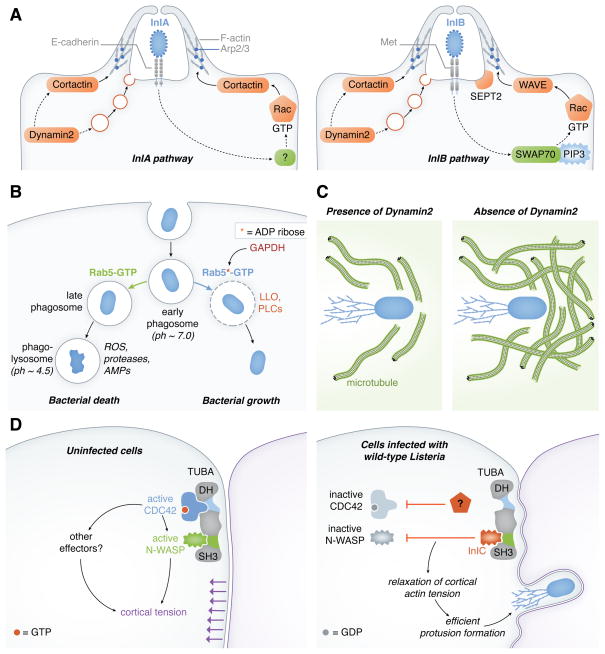
Figure 2. Subversion or inhibition of host GTPases by Listeria.
(A). GTPases involved in InlA- or InlB-mediated bacterial entry. Human GTPases and their effectors known to participate in internalization of Listeria are colored in orange. Solid arrows indicate known effects on bacterial entry, whereas dashed lines denote possible effects. InlA-dependent entry involves the GTPase Rac1 and the NPF cortactin, which promote actin polymerization through the Arp2/3 complex. ‘?’ indicates that the GEF involved in Rac1 activation is unknown. InlB-mediated entry is promoted by Rac1 and its NPFs WAVE and cortactin. The GEF SWAP70 is required for Listeria entry, and possibly activates Rac1 during this process. The septin GTPase SEPT2 is recruited to sites of Listeria entry and is required for bacterial uptake. The GTPase Dynamin 2 is needed for both InlA- and InlB- mediated internalization. Possible modes of action of Dynamin 2 include recruitment of cortactin, stimulation of focal exocytosis, or scission of the developing phagosome (not shown). For the sake of simplicity, some host GTPases that regulate Listeria entry, such as Cdc42, Arf6 and SEPT11, are not depicted in this diagram. ‘PIP3’ indicates phosphatidylinositol 3,4,5-tris phosphate. (B). Control of phagosomal survival by Rab5 GTPase. Rab5-GTP promotes the maturation of early phagosomes to phagolysosomes, contributing to killing of internalized bacteria (left pathway). By producing the protein GAPDH, Listeria diminishes Rab5-GTP levels and allows phagosomal survival until escape by LLOs and PLCs occurs (right pathway). GAPDH ADP-ribosylates Rab5, thereby inhibiting nucleotide exchange and maintaining Rab5 in a GDP bound state. ‘ROS’ indicates reactive oxygen species, and ‘AMPs’ denotes antimicrobial peptides. (C). Regulation of bacterial motility by Dynamin 2. The density of microtubules increases in cells depleted for Dynamin 2, resulting in impaired actin-based motility of Listeria. (D). Model of the mechanism of relief of host inhibition of Listeria spread by InlC and other bacterial factors. The images in each panel represent XY views (cross sections) at the level of the tight junction. Left panel: Without InlC or other bacterial proteins, cortical tension at the plasma membrane is generated by [1] The Tuba SH3 domain binding to N-WASP to recruit N-WASP to the plasma membrane and/or activate N-WASP, and [2] Tuba-mediated stimulation of Cdc42 to activate N-WASP and/or other effectors (?). Right panel: In cells infected with wild-type Listeria, the bacterial protein InlC blocks N-WASP binding to Tuba SH3 domain. In addition, an unknown bacterial factor ‘?’ reduces Cdc42 activity. The combined effects of InlC and the unidentified microbial factor reduce cortical tension and allow efficient protrusion formation by Listeria.