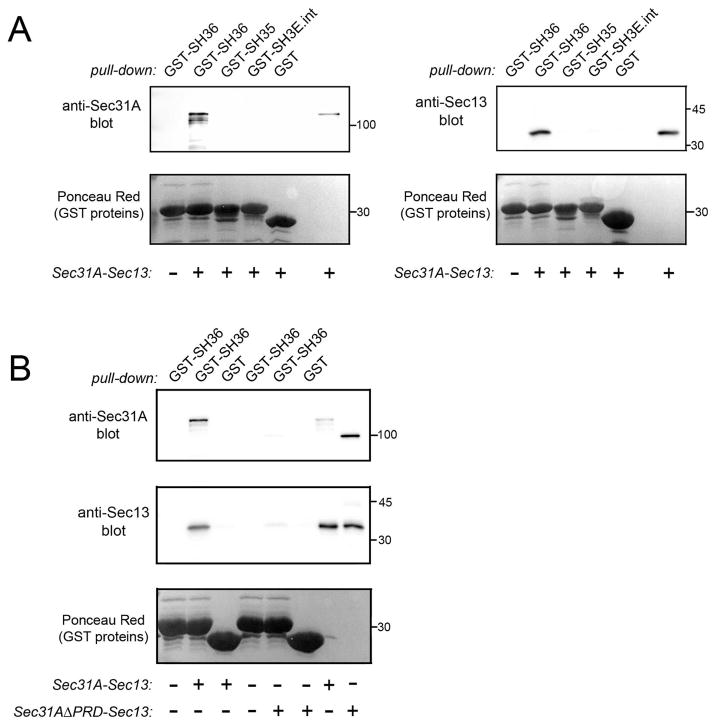
Figure 2. The Tuba SH36 domain interacts directly with the Sec31A-Sec13 complex.
A. Binding of SH36 to wild-type Sec31A complexed with Sec13. Purified Sec31A-Sec13 complex was incubated with GST-SH36 and binding was assessed in co-precipitation assays as described in the Experimental Procedures. As negative controls, GST alone or GST fused to the SH35 domain of Tuba (GST-SH35) or the SH3E domain of intersectin-l (GST-SH3E.int) were used. Binding was detected by probing precipitates with antibodies against Sec31A (left panel) or anti-Sec13 (right panel). In the last lane, 100 ng of Sec31A-Sec13 protein was loaded as a control for antibody reactivity. B. Experiments with Sec31A deleted for its proline-rich domain (Sec31AΔPRD) complexed with Sec13. Co-precipitation experiments were performed with purified Sec31A-Sec13 or Sec31A-ΔPRD-Sec13 complexes and the indicated GST fusion proteins. The last two lanes contain 100 ng of purified Sec31A-Sec13 or Sec31AΔPRD-Sec13 complexes. Experiments in A and B were performed 3 times with similar results.