Abstract
AIM: To characterize the anticancer function of cytokine-induced killer cells (CIK) and develop an adoptive immunotherapy for the patients with primary hepatocellular carcinoma (HCC), we evaluated the proliferation rate, phenotype and the antitumor activity of human CIK cells from healthy donors and HCC patients in vitro and in vivo.
METHODS: Peripheral blood mononuclear cells (PBMC) from healthy donors and patients with primary HCC were incubated in vitro and induced into CIK cells in the presence of various cytokines such as interferon-gamma (IFN-γ), interleukin-1 (IL-1), IL-2, and monoclonal antibody (mAb) against CD3. The phenotype and characterization of CIK cells were identified by flow cytometric analysis. The cytotoxicity of CIK cells was determined by 51Cr release assay.
RESULTS: The CIK cells were shown to be a heterogeneous population with different cellular phenotypes. The percentage of CD3+CD56+ positive cells, the dominant effector cells, in total CIK cells from healthy donors and HCC patients, significantly increased from 0.1%-0.13% at day 0 to 19.0%-20.5% at day 21 incubation, which suggested that the CD3+CD56+ positive cells proliferated faster than other cell populations of CIK cells in the protocol used in this study. After 28 day in vitro incubation, the CIK cells from patients with HCC and healthy donors increased by more than 300-fold and 500-fold in proliferation cell number, respectively. CIK cells originated from HCC patients possessed a higher in vitro antitumor cytotoxic activity on autologous HCC cells than the autologous lymphokine-activated killer (LAK) cells and PBMC cells. In in vivo animal experiment, CIK cells had stronger effects on the inhibition of tumor growth in Balb/c nude mice bearing BEL-7402-producing tumor than LAK cells (mean inhibitory rate, 84.7% vs 52.8%, P < 0.05) or PBMC (mean inhibitory rate, 84.7% vs 37.1%, P < 0.01).
CONCLUSION: Autologous CIK cells are of highly efficient cytotoxic effector cells against primary hepatocellular carcinoma cells and might serve as an alternative adoptive therapeutic strategy for HCC patients.
INTRODUCTION
Cytokine-induced killer (CIK) cells are the major histocompatibility complex-unrestricted cytotoxic lymphocytes and generated by incubation of peripheral blood monocytes (PBMC) in the presence of various types of cytokines such as CD3 monoclonal antibody, interleukin-2 (IL-2), interleukin-1 (IL-l) and interferon-gamma[1]. CIK cells are the population of heterogenous effector cells possessing enhanced cytotoxicity and a higher proliferation rate as compared with lymphokine-activated killer (LAK) and tumor infiltrating lymphocytes (TIL) cells. The high anti-tumor activity of CIK cells is mainly due to the high proliferation of double CD3+ and CD56+ positive cells[2-5]. Some reports indicated that CIK cells, other than LAK and TIL cells, can be efficiently employed as an adjuvant in anticancer immunotherapeutic strategy for the eradication of residual cancer cells and prevention or delay of tumor relapse[6-9].
Hepatocellular carcinoma (HCC) is the malignant transformation of hepatocytes and is a common complication of chronic hepatitis mainly caused by hepatitis B virus (HBV) and hepatitis C virus (HCV) infection in China[10]. The mechanisms underlying the malignant transformation of hepatocytes are not well defined. Many driving factors including the persistent hepatitis B or C virus infections, host immunological and genetic factors might have been involved in the process. For example, a failure to mount an efficient immune response to HCC cells, either because of selective defects or immune tolerance in the host immune system or because of tumor interference with a function (s) of immune cells, could account for the inability of HCC patients to block the pathogenesis of HCC occurrence[11-18]. In attempt to characterize the anticancer function of CIK cells and develop an alternative adoptive immunotherapy for the patients with primary HCC, we evaluated the proliferation rate and phenotype of CIK cells from healthy donors and primary HCC patients. Furthermore, we compared the cytotoxic activity and antitumor effects of major effector CIK cells with double CD3+ and CD56+ positive markers against the primary and secondary HCC in in vitro cell culture system and in vivo tumor-bearing mice model.
MATERIALS AND METHODS
Reagents and cell lines
RPMI1640 and DMEM medium, recombinant human IL-1a, IL-2, interferon gamma (IFN-γ), TNF-α and monoclonal antibody against the CD3 surface antigen (mAb CD3) were all purchased from Gibco Co. The Flt-3 ligand, mouse anti-human FITC-conjugated CD3 and CD56 monoclonal antibodies were obtained from BD-Pharmingen and Fetal calf serum (FCS) from Hyclone. BEL7402 is human HCC cell line kept in liquid nitrogen in our laboratory.
Cell separation
The patients with primary HCC and healthy donors were submitted to cytopheresis after their writing contents were signed. An enriched peripheral blood mononuclear cells (PBMC) product was collected using the specific program of the Cobe Spectra blood separator. Cells were resuspended in phosphate buffered saline (PBS) without calcium and magnesium. The separation of PBMC was performed as previously described[2,19-21]. The concentrated PBMC cells were used immediately for CIK cell culture.
Generation of cytokine-induced killer (CIK) cells
CIK cells were generated as described previously[2,3,22,23]. Briefly, non-adherent Ficoll-separated human peripheral blood mononuclear cells were prepared and incubated in RPMI1640 medium containing 100 mL·L-1 FCS and various types of cytokines added according to the reported protocol with minor modifications[23]. The final concentrations of the cytokines and antibody added were as follows: IL-2, 1000 × 103 U·L-1; IL-1, 100 × 103 U·L-1; IFN-γ, 100 × 103 U·L-1; mAb CD3, 50 μg·L-1. Cells were incubated at 37 °C in a humidified atmosphere of 50 mL·L-1 CO2 and fed every 3 days in fresh complete medium with 100 mL·L-1 FCS and various types of cytokines at 0.5 × 109 cells·L-1.
Immunofluroescent staining of effector cells
Starting PBMC, LAK or CIK effector cells were stained with various types of FITC- or PE-conjugated mouse mAb’s against human surface antigens. Antibodies used included anti-CD3, anti-CD4, anti-CD16, anti-CD56 and anti-TCR-α/β, anti-TCR-γ/δ (all from Becton Dickinson, Beijing, China). Five × 105 of cells were incubated with a optimal amount of antibody at 4 °C for 30 min. To remove excessive antibody, the incubation mixture was centrifuged at 250 × g for 10 min. The pellet was resuspended in PBS containing 2 mL·L-1 of human AB serum and treated as described before[21]. Stained cells were washed and analyzed with FACScaliber (Becton Dickinson) in our laboratory.
Isolation of primary HCC cells from patients
Fresh liver cancer tissues were obtained from the HCC patients at the department of Surgery in our hospital and immediately immersed in sterilized PBS (pH7.0) solution containing penicillin, 100 × 103 U·L-1; streptomycin, 100 × 103 U·L-1 and gentamycin, 500 × 103 U·L-1 for 1 h at room temperature, then washed with PBS for three times. The liver cancer tissues were cut into small pieces (1-2 mm3 in size), and digested with 37 °C-prewarm serum-free medium with 1.25 g·L-1 of collagenase-V for 2 h. In order to separate them into single cell suspension, all the digested pieces of tissues were filtered through the 200 mesh of sterilized copper wire bag. The single cell suspension was further isolated by Percoll gradient centrifugation. Finally, the resuspended cells in DMEM medium with 200 mL·L-1 FCS were the primary HCC cells.
Cytotoxic effects of CIK cells by 51C release assay
51Cr release cytotoxic assays were performed as reported previously[24]. Briefly, one × 106 of human primary HCC cells were incubated with culture medium containing 7.4MBq of 51Cr solution at 37 °C for one hour. The labeling cells were washed with PBS solution for three times and added to 96-well culture plates at 2 × 104 cells/well. CIK cells were incubated with tumor cells at various ratios of effector cells to target cells as 6.25:1, 12.5:1, 25:1 and 50:1. After 4 h incubation, all cells were collected by centrifugation and an aliquot of supernant was counted in a gamma counter. The percentage of specific release was calculated as described[25].
Inhibitory Effects of CIK cells on HCC in tumor-bearing nude mice models
In pilot experiments, 1.5 × 106 of BEL-7402 cells injected subscapularly could produce solid tumor in Balb/c nude mice at the tenth day with 100% of incidence rate. The Balb/c nude mice used in the study were randomly divided into four groups treated with physical solution, PBMC, LAK and CIK cells, respectively. The nude mice received 3.0 Gy of whole body irradiation and injected subscapularly with 5 × 106 BEL-7402 cells, which were at the stage of exponential growth (designated as day 0). On day 1, 0.25 mL of 1 × 107 effector cells such as CIK, PBMC or LAK cells were injected locally where tumor cells were inoculated for 6 consecutive days. In controls, the nude mice received 0.25 mL physical solution. The size of tumor was recorded every other day. The solid tumors were peeled off after the nude mice were dislocated by killing them on the 35th day.
Statistical analysis
The Wilcoxon matched-pairs test was used to analyze for statistical significance. The P value less than 0.05 was considered as significant difference.
RESULTS
Proliferation and phenotype of CIK cells
Figure 1A and Figure 1B show the proliferation of CIK cells from healthy donors and patients with primary HCC at different incubation days. During cell generation, there was a steady increase in both the absolute number and the percentage of CD3+CD56+ cells, e.g. the percentage of CD3+CD56+ cells was 7.5% on 14 d and 51.3% on 56 d of in vitro incubation, respectively. After 14 d in vitro incubation, the number of total incubated CIK cells increased significantly (500 fold from 8.07 × 105 to 1.02 × 108). The majority (as high as 82% ± 6.4%) of these cells were positive for TCR-α/β. Cells expressing TCR-γ/δ were relatively rare (4.5% ± 2.6%). The proliferation capability of PBMC obtained from normal donors was slightly higher than that of PBMC obtained from the HCC patients. The percentage of double CD3+CD56+ positive cells varied during CIK cell generation. The percentage of CD3+CD56+ positive cells in total CIK cells from healthy donors and HCC patients significantly increased from 0.1%-0.13% at day 0 to 18.95%-20.5% at day 21, which suggested that the CD3+CD56+ positive cells proliferated faster than other populations of CIK cells in the protocol used in this study.
Figure 1.
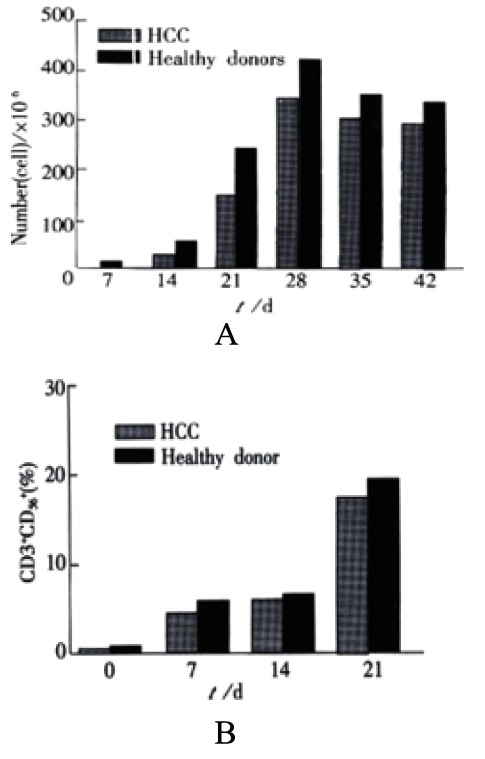
A: Proliferation of CIK cells in vitro culture system. B: The percentages of double CD3+CD56+ positive cells at various incubation time in vitro culture system.
In peripheral blood, 24.2% of CD56+ cells coexpressed CD3+ as compared to 36.2% in LAK cell cultures and 76% in CIK cell cultures (Figure 2). Conversely, only 17.8% and 42.1% of CD3+ cells in peripheral blood and LAK cells coexpressed CD56+, whereas 76.6% of CIK CD3+ cells coexpressed CD56+. At d 28 of CIK cell generation, the percentage of CD3+ cells coexpressing CD56+ increased to 82.4% (Table 1).
Figure 2.
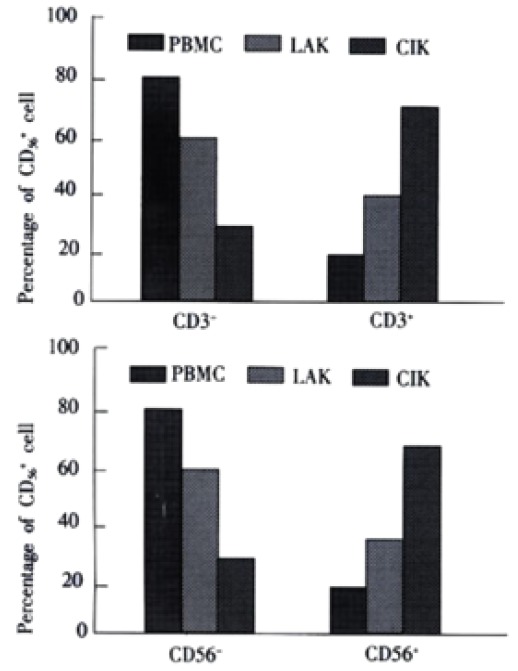
Subsets of CIK cells from patients with primary HCC. CIK cells were counterstained for CD3 and CD56 at day 12 of CIK generation and the percentage of positively stained cells was determined by flow cytometry. PBMC and LAK were stained like CIK cells. LAK cells were used at day 6 of generation. Upper. CD56+ subsets of CIK cells, LAK, PBMC. Subsets of CD56+ cells were compared to the total number of CD56+ cells. The figure represents data from three different experiments. Results are presented as mean value ± SEM. Lower. CD3+ subsets of CIK cells, LAK, PBMC. Subsets of CD3+ cells were compared to the total number of CD3+ cells. The figure represents data from three different experiments. Results are presented as mean value ± SEM.
Table 1.
Phenotype of CIK cells and cytotoxic activity of CIK subsets
| Subset | Percentage positively of CIK | LU/106 cell stained cells | Cell number × 106 | Total LU per culture |
| CIK | 43.6 ± 4.8 | 712 ± 24.3 | 29, 074 | |
| TCR-α/β | 82.0 ± 6.4 | 20.6 ± 3.8 | 583 ± 41.6 | 12, 088 |
| CD56 | 30.4 ± 5.6 | 78.4 ± 6.9 | 214 ± 70.3 | 13, 948 |
| CD16 | 15.8 ± 5.4 | 60.4 ± 6.0 | 89 ± 40.9 | 4106 |
Note: Total LU per culture was calculated by multiplying the number of LU per million cells by the total number of cells.
Effect of different sera on CIK proliferation
When CIK cells were incubated in RPMI1640 medium containing 100 mL·L-1 of fetal calf serum or 100 mL·L-1 human AB serum or free-serum medium, respectively, they exhibited different growth curves (Figure 3). CIK cells had the highest proliferation rate in human AB serum-containing medium, the lowest in serum-free medium. The proliferated numbers of CIK cells increased by 500 fold in human AB serum-containing medium, 450 fold increase in fetal calf serum-containing medium, and 50-60 fold in free-serum medium, respectively.
Figure 3.
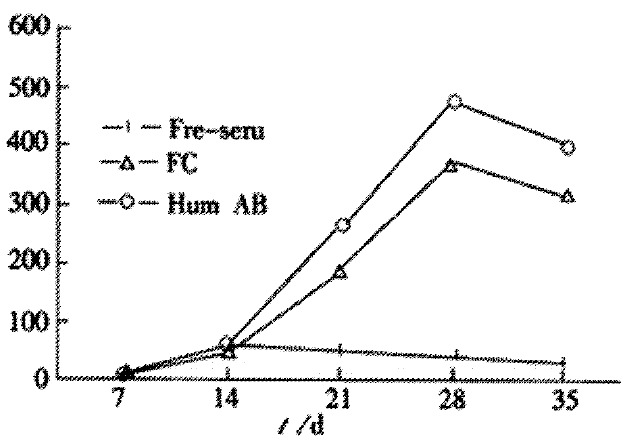
Effects of different culture system on growth of CIK cells
Cytotoxic activity of CIK cells on primary HCC cells
The CIK cell subpopulations were tested for cytotoxicity against the HCC cells as measured by 51Cr release. The primary HCC cells were used as the target cells and the CIK cells from the same patients with HCC as the effector cells. Both of them at various ratios of effector cells to target cells were mixed together for evaluation of the cytotoxic activity of CIK cells. CD3+CD56+ T cells were identified to be the major cytolytic effectors, as previously reported, the CD3+CD56+ subpopulation of CIK cells exhibited maximum 51Cr release cytotoxicity toward target cells (Figure 4) with the increased ratio of effector and target cells, the cytotoxic effects of CIK cells correspondingly became stronger. In controls, PBMC cells only showed a weaker cytotoxic effect on primary HCC cells compared with CIK cells, and maintained at a lower platform level even if the ratio of effector and target cells increased.
Figure 4.
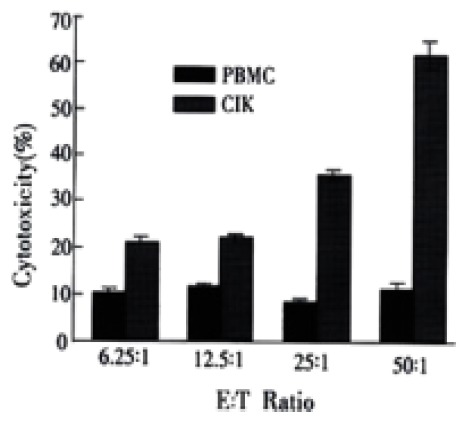
Cytotoxic activity of CIK, PBMC cells against primary HCC cells
Inhibitory effects of CIK cells on HCC in tumor-bearing nude mice
Inhibitory effects of CIK cells on HCC cells in tumor-bearing nude mice are summarized in Table 2. In CIK-treated group, 15 nude mice were inoculated with tumor cells and received treatment of CIK cells for six day consecutively. Tumorgenesis was found in ten of fifteen (66.7%) nude mice. However, tumorgenesis was formed in all the mice in PBMC and LAK treated groups. Furthermore, the tumor size was smaller and limited in the mice of CIK-treated group as compared with those in the mice of PBMC- and LAK-treated groups. There was an obvious difference between the CIK group and LAK group (P < 0.05), and between CIK group and PBMC group (P < 0.01), which suggest that CIK cells have a great priority of inhibitory effects on tumorgenesis in tumor-bearing nude mice as compared with LAK cells.
Table 2.
Inhibitory effects of CIK, PBMC and LAK cells on HCC cells in tumor-bearing nude mice
| Group | n | Tumor/g | Tumorgenesis (%) | Inhibitory rate (%) |
| Control | 9 | 2.10 ± 0.41 | 100 | 0 |
| PBMC | 11 | 1.32 ± 0.27 | 100 | 37.1 |
| LAK | 11 | 0.99 ± 0.33 | 100 | 52.8 |
| CIK | 15 | 0.32 ± 0.31 | 66.7 | 84.7ab |
P < 0.05 vs LAK group,
P < 0.01 vs PBMC group.
DISCUSSION
In this study, we found that aotulogous CIK cells had a higher proliferation rate and enhanced cytotoxic activity compared with lymphokine-activated killer cells[5,26,27]. The CIK cells from the primary HCC patients had a lower pro liferation ability than that from the healthy donors though no obvious difference was found in the phenotype and components of CIK cells between the two groups. Interestingly, the cytotoxic activity of CIK cells from healthy donors was proved to be stronger than that from the patients with primary HCC. This results might reflect the functions of CIK cells in HCC patients is probably inhibited to some content by itself, which is consistent with the fact that there is a decreased function of peripheral blood dendritic cells in patients with primary HCC[28,29]. Whether the dysfunctional dendritic cells led to the decreased proliferation and function of CIK cells? Martin et al[5] reported that CIK cells significantly increased the cytotoxic activity after co-cultured with aotulogous dendritic cells, and they considered this phenomenon to be induced by IL-12 cytokine secretion from dendritic cells and cell-cell interactions between the two population.
Though LAK cells recognize and kill target cells by a non-MHC restricted mechanism in the same way like CIK cells, the CIK cells were found to have the greatest lytic activity[5]. Our results suggested that the CIK effector cells are the subpopulation coexpressing CD3+CD56+ phenotypes, which supported the facts that CIK cells possessed a higher proliferation rate and higher antitumor cytotoxic activity in vitro than LAK cells[26,30,31].
Immunological effector cells involved in cell-mediated cytotoxicity, such as CIK cells, cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, possess cytoplasmic granules that are involved in target cell death. These granules contain a pore-forming protein called perforin or cytolysin, a family of serine esterases (granzymes), lysosomal enzymes, and proteoglycan molecules. The cytolysis of tumor cells produced by CIK effector cells might be mediated by the local direct exocytosis of cytoplasmatic granules that penetrate the cell membrane of the bound target cell. Stimulation of CIK cells with tumor cells or anti-CD3 mAb is sufficient for exocytosis of cytotoxic material. The detailed mechanism by which these effector cells recognize tumor cell targets has remained elusive. However, leukocyte function associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) appears to be involved. CIK cells do not express the CD16 (Fc receptor) surface molecule, and therefore do not participate in antibody-dependent cellular cytotoxicity (ADCC). The CD3+CD56+ cell subpopulation is derived from T cells. The cytotoxic effects of CIK cells against tumor cell targets are blocked by antibodies against the adhesion receptor LFA-1 and its counter receptor, ICAM-1, indicating that the adhesion molecule LFA-1 responsible for cellular interaction is necessary for CIK cell-mediated cytotoxicity. Cognate tumor cell targets could also induce BLT esterase release from CIK cells. These observations showed that the cellular interaction involving LFA-l is important for cytolysis of target cells by the CIK cells. The mechanism of exocytosis of cytoplasmic granules leading to cytolysis of the target cell is still unclear. Based on the previous reports, the pathway by which CIK cells can kill target cells, is probably dependent on LFA-l, which probably leads to a granule-dependent cytolysis and is usually the dominant one and accounts for CIK cell cytotoxicity against HCC target cells mediated by CIK recognition structures. Identification of the putative CIK receptors for tumor cell targets as well as other cell surface molecules involved in these cytotoxicity mechanisms will be extremely useful for further understanding of non-MHC-restricted lymphocyte cytotoxicity against tumor cells[5,21,32,33].
Our study showed that the human CIK cells could eradicate 84.7% of the established human hepatoma tumor in nude mice and had no toxic side effects, which is consistent with the reports described before[5,30,31]. In addition, CIK cells have an important property of efficiently killing the resistant tumor cells with overexpression of p-glycoprotein[33,34]. Recent studies proved that the activity of CIK cells killing tumor cells in a non-MHC restricted way could be enhanced by co-culture with dendritic cells or by using the bispecific antibody. In summary, CIK cells may have a major impact on the adoptive immunotherapeutic protocols for patients with primary HCC[35,36].
Footnotes
Supported by Science and Technology Development Foundation of Beijing Institute of Infectious Diseases, No. 01Z094
Edited by Ma JY
References
- 1.Zoll B, Lefterova P, Csipai M, Finke S, Trojaneck B, Ebert O, Micka B, Roigk K, Fehlinger M, Schmidt-Wolf GD, et al. Generation of cytokine-induced killer cells using exogenous interleukin-2, -7 or -12. Cancer Immunol Immunother. 1998;47:221–226. doi: 10.1007/s002620050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du QY, Wang FS, Xu DP, Liu H, Lei ZY, Liu MX, Wang YD, Chen JM and Wu CT. Cytotoxic effects of CIK against hepatocellular carcinoma cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:863–866. [Google Scholar]
- 3.Maki G. Ex vivo purging of stem cell autografts using cytotoxic cells. J Hematother Stem Cell Res. 2001;10:545–551. doi: 10.1089/15258160152509154. [DOI] [PubMed] [Google Scholar]
- 4.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 5.Märten A, Renoth S, von Lilienfeld-Toal M, Buttgereit P, Schakowski F, Glasmacher A, Sauerbruch T, Schmidt-Wolf IG. Enhanced lytic activity of cytokine-induced killer cells against multiple myeloma cells after co-culture with idiotype-pulsed dendritic cells. Haematologica. 2001;86:1029–1037. [PubMed] [Google Scholar]
- 6.Shi Y, Yu J, Cen X, Zhu P, Ma M. [Large-capacity expanded cytoline-induced killer cells and its cytotoxic activity] Shengwu Yixue Gongchengxue Zazhi. 2001;18:94–96. [PubMed] [Google Scholar]
- 7.Alvarnas JC, Linn YC, Hope EG, Negrin RS. Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2001;7:216–222. doi: 10.1053/bbmt.2001.v7.pm11349808. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman DM, Gitlitz BJ, Belldegrun A, Figlin RA. Adoptive cellular therapy. Semin Oncol. 2000;27:221–233. [PubMed] [Google Scholar]
- 9.Andreesen R, Hennemann B, Krause SW. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol. 1998;64:419–426. doi: 10.1002/jlb.64.4.419. [DOI] [PubMed] [Google Scholar]
- 10.Zhou HG, Gu GW. Study of molecular epidemiology in hepatocellular carcinoma in China. Shijie Huaren Xiaohua Zazhi. 1998;6:432–434. [Google Scholar]
- 11.Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendritic cells against human hepatoma cell line. Shijie Huaren Xiaohua Zazhi. 1999;7:191–193. [Google Scholar]
- 12.Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells induce the enhancement of immune reactions against hepatocellular carcinoma cells in vitro. Shijie Huaren Xiaohua Zazhi. 1999;7:161–163. [Google Scholar]
- 13.Ma CH, Sun WS, Cao YL, Zhang LN, Song J. Coinhibitory effect of recombinant tumor necrosis factor α and mutant interleukin-2 on H7402. Huaren Xiaohua Zazhi. 1998;6:97–98. [Google Scholar]
- 14.He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of alpha-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol. 1999;5:111–115. doi: 10.3748/wjg.v5.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Ku JL, Park YJ, Lee KU, Kim WH, Park JG. Establishment and characterization of four human hepatocellular carcinoma cell lines containing hepatitis B virus DNA. World J Gastroenterol. 1999;5:289–295. doi: 10.3748/wjg.v5.i4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong XB, Yang ZK, Liang LJ, Huang JF, Lin HL. Overexpression of P-glycoprotein in hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2000;6:134–135. doi: 10.3748/wjg.v6.i1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang DH, Zhang MQ, Du J, Xu C, Liang QM, Mao JF, Qin HR, Fan ZR. Inhibitory effect of IGF- II antisense RNA on malignant phenotype of hepatocellular carcinoma. World J Gastroenterol. 2000;6:266–267. doi: 10.3748/wjg.v6.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment: a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol. 2000;6:348–352. doi: 10.3748/wjg.v6.i3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74:51–56. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- 20.Finke S, Trojaneck B, Lefterova P, Csipai M, Wagner E, Kircheis R, Neubauer A, Huhn D, Wittig B, Schmidt-Wolf IG. Increase of proliferation rate and enhancement of antitumor cytotoxicity of expanded human CD3+ CD56+ immunologic effector cells by receptor-mediated transfection with the interleukin-7 gene. Gene Ther. 1998;5:31–39. doi: 10.1038/sj.gt.3300560. [DOI] [PubMed] [Google Scholar]
- 21.Ren H, Xing SX, Xu HW, Song YH, Shang XZ, Zhou GS, Tian JX, Li DJ. Initial Study on proliferation of CIK cells and their antitumor activity in vitro and in vivo. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 1999;6:17–21. [Google Scholar]
- 22.Zoll B, Lefterova P, Ebert O, Huhn D, Von Ruecker A, Schmidt-Wolf IG. Modulation of cell surface markers on NK-like T lymphocytes by using IL-2, IL-7 or IL-12 in vitro stimulation. Cytokine. 2000;12:1385–1390. doi: 10.1006/cyto.2000.0733. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M, Takeya M, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81:1009–1016. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, Christensson B, Dilber MS. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001;62:1092–1098. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 25.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92:3318–3327. [PubMed] [Google Scholar]
- 26.Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hepatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673–675. [Google Scholar]
- 27.Xiao LF, Luo LQ, Zou Y, Huang SL. Study of the phenotype of PBLs activated by CD28/CD80 and CD2/CD58 and acting with hepatoma cells and the restricted usage of TCR Vβ gene subfamily. Shijie Huaren Xiaohua Zazhi. 1999;7:1044–1046. [Google Scholar]
- 28.Kakumu S, Ito S, Ishikawa T, Mita Y, Tagaya T, Fukuzawa Y, Yoshioka K. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2000;15:431–436. doi: 10.1046/j.1440-1746.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- 29.Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31:323–331. doi: 10.1016/s0168-8278(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 30.Flieger D, Kufer P, Beier I, Sauerbruch T, Schmidt-Wolf IG. A bispecific single-chain antibody directed against EpCAM/CD3 in combination with the cytokines interferon alpha and interleukin-2 efficiently retargets T and CD3+CD56+ natural-killer-like T lymphocytes to EpCAM-expressing tumor cells. Cancer Immunol Immunother. 2000;49:441–448. doi: 10.1007/s002620000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller M, Scheffold C, Lefterova P, Huhn D, Neubauer A, Schmidt-Wolf IG. Potential of autologous immunologic effector cells for prediction of progression of disease in patients with chronic myelogenous leukemia. Leuk Lymphoma. 1998;31:335–341. doi: 10.3109/10428199809059226. [DOI] [PubMed] [Google Scholar]
- 32.Verneris MR, Kornacker M, Mailänder V, Negrin RS. Resistance of ex vivo expanded CD3+CD56+ T cells to Fas-mediated apoptosis. Cancer Immunol Immunother. 2000;49:335–345. doi: 10.1007/s002620000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, Tsuruo T, Huhn D, Negrin RS. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol. 1996;169:85–90. doi: 10.1006/cimm.1996.0094. [DOI] [PubMed] [Google Scholar]
- 34.Wang FS, Kobayashi H, Liang KW, Holland JF, Ohnuma T. Retrovirus-mediated transfer of anti-MDR1 ribozymes fully restores chemosensitivity of P-glycoprotein-expressing human lymphoma cells. Hum Gene Ther. 1999;10:1185–1195. doi: 10.1089/10430349950018175. [DOI] [PubMed] [Google Scholar]
- 35.Toporski J, Gorczyńska E, Kalwak K, Turkiewicz D, Nowakowska B, Ryczan R, Boguslawska-Jaworska J. Double haploidentical transplantation of hematopoietic progenitor cells in a boy with myelodysplastic syndrome. Pediatr Hematol Oncol. 1999;16:257–261. doi: 10.1080/088800199277326. [DOI] [PubMed] [Google Scholar]
- 36.Lefterova P, Märten A, Buttgereit P, Weineck S, Scheffold C, Huhn D, Schmidt-Wolf IG. Targeting of natural killer-like T immunologic effector cells against leukemia and lymphoma cells by reverse antibody-dependent cellular cytotoxicity. J Immunother. 2000;23:304–310. doi: 10.1097/00002371-200005000-00003. [DOI] [PubMed] [Google Scholar]


