Abstract
AIM: To study the correlation between genetic polymorphism of cytochrome P450IIE1 (CYPIIE1) and fatty liver.
METHODS: Peripheral blood mononuclear cells were collected in 56 patients with fatty liver, 26 patients without fatty liver and 20 normal controls. Then PCR-RFLP was used to analyze genetic polymorphism of CYPIIE1 in monocytes on the region of Pst I and Rsa I.
RESULTS: The frequency of homozygotic C1 gene in patients with alcoholic fatty liver (28.6%), obese fatty liver (38.5%), or diabetic fatty liver (33.3%) was significantly lower than that of the corresponding patients without fatty liver (100%, 100% and 80% respectively), while the frequency of C2 genes, including C1/C2 and C2/C2, was significantly higher (71.4%/0%, 61.5%/0%, and 66.7%/20%) (P < 0.01). The frequency distribution of the above genes of non-fatty liver patients (100%/0, 100%/0, and 80%/20%) was not significantly different from that of the normal controls (85%/15%) (P > 0.05).
CONCLUSION: The genetic polymorphism of CYPIIE1 on the position of Pst I and Rsa I is related to the susceptibility of fatty liver. Besides, C2 gene may play a key role in the pathogenesis of fatty liver.
INTRODUCTION
Cytochrome P450 (CYP) is a group of isoenzymes encoded by genes with similar structure and function, whose molecular weight is 40-60KD. CYP is a kind of monooxygenase, located in the smooth endoplasmic reticulum of cells. According to the similarity of amino acid sequence, CYP is divided into CYPI, CYPII, CYPIII, and CYPIV gene families. The subfamily is named alphabetically, and every enzyme is named in number order. Cytochrome P450IIE1 (CYPIIE1) is a N-nitrosodimethylamine demethylase, which is mainly expressed in the liver with an evident racial and individual difference in activity. It not only takes part in the metabolism of drugs, but also activates a lot of precarcinogens and prepoison[1-4]. Human CYPIIE1 is located in 10q2403-qter. It is 1104Kb consisting of 9 extrons and 8 introns, encoding a 493-amino acid protein[5]. The polymorphism of CYPIIE1 gene is significantly different among races and individuals. It may be related to some genetic factors. CYPIIE1 has 6 restriction fragment length polymorphisms (RFLP), among which 5’-flanking region of Pst I and Rsa I affects CYPIIE1 expression at the transcription level. C2 allelic gene can enhance the transcription, which causes the different activities of CYPIIE1[6-11].
Fatty liver is common and is resulted frequently from alcohol excess, obesity, diabetes or drugs[12-17]. Its pathogenesis remains unclear. Studies on the relationship between genetic polymorphisms of CYPIIE1 and the development of alcoholic fatty liver has been reported, but the results are disputable[17-21]. In this study, we used PCR-RFLP to study the relationship between genetic polymorphisms of CYPIIE1 and alcoholic or non-alcoholic fatty liver.
MATERIALS AND METHODS
Reagents
Heparin and lymphocyte separation medium were purchased from Tianjin Hematologic Institution of Chinese Academy of Medical Sciences. The primers of CYPIIE1, PCR buffer, dNTP, and Taq enzyme were obtained from Roche (America). Restriction endonucleases (Pst I and Rsa I), their buffer, and pUC19-DNA marker were obtained from MBI Ferments.
Patients and controls
From October 1998 to January 2001, 82 patients from several hospitals in Jilin Province were studied, among them 28 cases had alcoholic fatty liver, 8 cases had alcoholism but no liver disease, 13 cases had obese fatty liver, 8 cases had obesity but no fatty liver, 15 cases had diabetic fatty liver, and 10 cases had diabetes but no fatty liver. Twenty health blood donors were used as normal controls. The standard of alcoholism for female was drinking alcohol > 40 g/d, for male drinking alcohol > 80 g/d, for at least 5 years. The age and sex distribution of the two groups were similar. Five mL venous blood was taken from every person and anticoagulated with heparin.
PCR-RFLP
Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation. Then DNA was extracted. The two primers of CYPIIE1 were 5’-ccagtcgagtctacattgtca-3’ (1370-1349) and 5’-ttcattctgtcttctaactgg-3’ (999-978) respectively. PCR was conducted for 40 cycles with denaturing at 94 °C for 1 min, annealing at 50 °C for 1 min, extending at 72 °C for 1 min, and then designed to extend at 72 °C for 10 min. The PCR products were digested with Pst I or Rsa I at 37 °C for 2 h, then loaded onto a 20 g/L agarose gel for electrophoresis. At last, the DNA fragments were observed under ultraviolet lamp.
Statistical analysis
Analysis of data was performed using χ² test. P < 0.05 was considered to be statistically significant.
RESULTS
Genetic polymorphism of CYPIIE1
The PCR products were fragments of 410 bp (Figure 1). After digestion with restriction enzyme Pst I or Rsa I, CYPIIE1 was divided wild type homozygote group (C1/C1, type A), heterozygote group (C1/C2, type B) and mutant homozygote group (C2/C2, type C) (Figure 2). C1 referred to wild type gene (PstI+, RsaI-), and C2 referred to mutant gene (PstI-, RsaI+).
Figure 1.
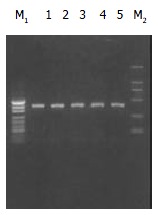
Electrophoregram of PCR products (Lanes 1-5). The signals of pUC19-DNA marker (M1) present 489 bp, 404 bp, 331 bp, 242 bp, 190 bp, 147 bp and 110 bp from up to bottom. The signals of DL2000-DNA marker (M2) present 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp and 100 bp from up to bottom.
Figure 2.
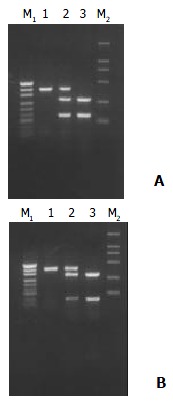
After digested with restriction enzyme PstI (A) or RsaI (B), CYPIIE1 was divided into three types, namely wild type homozygote (C1/C1) (Lane 1), heterozygote (C1/C2) (Lane 2) and mutant homozygote (C2/C2) (Lane 3). C1 means wild type gene (PstI+, RsaI-), and C2 mutant gene (PstI-, RsaI+). pUC19-DNA marker (M1) and DL2000-DNA marker (M2).
Genotype distribution
The genotype distribution of each group of patients and controls are presented in Table 1.
Table 1.
Genotype distribution of each group of patients and controls
| Group | n | A (C1/C1) | B (C1/C2) | C (C2/C2) |
| Patients with alcoholic fatty liver | 28 | 8 | 14 | 6 |
| Patients with alcoholism but without liver diseases | 8 | 8 | 0 | 0 |
| Patients with obese fatty liver | 13 | 5 | 6 | 2 |
| Patients with obese but without fatty liver | 8 | 8 | 0 | 0 |
| Patients with diabetic fatty liver | 15 | 5 | 8 | 2 |
| Patients with diabetes but without fatty liver | 10 | 8 | 2 | 0 |
| Healthy controls | 20 | 17 | 3 | 0 |
Comparison of gene frequency
The frequency of homozygotic C1 gene in patients with alcoholic, obese, or diabetic fatty liver was significantly lower than that of patients with corresponding diseases but without fatty liver, while the frequency of C2 genes, including C1/C2 and C2/C2, was significantly higher (P < 0.01) (Table 2). Compared with healthy controls, the frequency of homozygotic C1 gene of the patients with alcoholic, obese, or diabetic fatty liver was apparently lower and the frequency of C2 gene was apparently higher (P < 0.01). At the same time, there was no obvious difference in homozygotic C1 gene or C2 gene between healthy controls and patients with alcoholism, obesity or diabetes but without fatty liver (P > 0.05).
Table 2.
Comparison of gene frequency of each group (%)
| Group | A | B | C | C2 frequency |
| Patients with alcoholic fatty liver | 28.6 | 50.0 | 21.4 | 71.4 |
| Patients with alcoholism but without liver diseases | 100 | 0 | 0 | 0 |
| Patients with obese fatty liver | 38.5 | 46.1 | 15.4 | 61.5 |
| Patients with obese but without fatty liver | 100 | 0 | 0 | 0 |
| Patients with diabetic fatty liver | 33.3 | 53.4 | 13.3 | 66.7 |
| Patients with diabetes but without fatty liver | 80 | 20 | 0 | 20 |
| Healthy controls | 85 | 15 | 0 | 15 |
DISCUSSION
There are three metabolic pathways of ethanol in the liver, alcohol dehydrogenase (ADH) in cytoplasm, microsomal ethanol oxidazing system (MEOS) in smooth endoplasmic reticulum, and catalase in peroxidase. The major component of MEOS is CYPIIE1[22,23]. When concentration of ethanol in blood and liver tissue is low, most of ethanol is oxidized by ADH. While for the alcoholism or the people in whose liver tissue the concentration of ethanol is higher than 10 mmol/L, the activation of MESO plays a key role in metabolism of ethanol. In the pathogenesis of alcoholic fatty liver, the function of CYPIIE1 was mainly to take part in lipid peroxidation (LOP) reaction and to increase the contents of microsomal oxygen and carbonyl free radical[16,24,25]. It has been proved in rat models that generation of microsomal oxygen and carbonyl free radical formed from oxidated ethanol was related to CYPIIE1[26,27]. It was presumed that these oxygen-derived free radicals might impair the liver by directly damaging liver cells, affecting the sensititity of the liver to LPO, and inducing antibody-dependent cytotoxic effect through combination with CYPIIE1 on the cell membrane[28-30]. Not every alcoholic abuser could inevitably induce liver injury. Iwahashi K and colleagues[31] reported that in the people who had C2 allele, CYPIIE1 activity was much higher, and metabolic ability on ethanol was much stronger. Our results showed that homozygotic C1 gene frequency of the patients with alcoholic fatty liver was significantly lower than that of the controls or the patients with alcoholism but without fatty liver, while C2 gene frequency was much higher. It suggested that C2 gene might induce the expression of CYPIIE1 and facilitate genesis of alcoholic fatty liver.
LPO also took part in the pathogenesis of non-alcoholic fatty liver induced by obese or diabetes[32,33]. Now the precise stimulator of LPO reaction is unclear. The expression of CYPIIE1 in the animal models and patients with nonalcoholic fatty liver might be related to the induction of acetone and fatty acid[34,35]. It has been proved that the level of CYPIIE1 in the rats with obesity was four times as high as that of the rats without obesity[36]. The rising concentration of fatty acid and pyruvic acid in the liver might be a risk factor in pathogenesis of nonalcoholic fatty liver. When the increased fatty acid concentration in blood of patients with obesity could not be oxidated by mitochondria completely, CYPIIE1 would have the ability to oxidize fatty acid and in turn is activated by it so as to strengthen the oxidation ability. During oxidation of fatty acid, high reactivity carbonyl free radicals would be produced, which then stimulated the production of LPO, at last injured the liver[37]. In patients with diabetes, the ketone bodies produced by the liver could not be totally utilized by extrahepatic tissues, and the level of acetone would rise in the liver. The acetone could not only induce CYPIIE1 activation, but also be resolved by it. A great many of free radicals were produced, thus injuring the liver[38]. Not all patients with obesity or diabetes suffer from fatty liver. Our results showed that C2 gene frequency in patients with obese fatty liver or diabetic fatty liver was obviously higher than that of the patients without fatty liver. In conclusion, C2 gene frequency in patients with alcoholic or nonalcoholic fatty liver is much higher than that of controls. So C2 gene may be important for the pathogenesis of fatty liver.
Footnotes
Edited by Zhang JZ and Wang XL
References
- 1.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6:724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez FJ, Gelboin HV. Transcriptional and posttranscriptional regulation of CYP2E1, an N-nitrosodimethylamine demethylase. Princess Takamatsu Symp. 1990;21:157–164. [PubMed] [Google Scholar]
- 3.Ramaiah SK, Apte U, Mehendale HM. Cytochrome P4502E1 induction increases thioacetamide liver injury in diet-restricted rats. Drug Metab Dispos. 2001;29:1088–1095. [PubMed] [Google Scholar]
- 4.Zangar RC, Benson JM, Burnett VL, Springer DL. Cytochrome P450 2E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact. 2000;125:233–243. doi: 10.1016/s0009-2797(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 5.Umeno M, McBride OW, Yang CS, Gelboin HV, Gonzalez FJ. Human ethanol-inducible P450IIE1: complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expression. Biochemistry. 1988;27:9006–9013. doi: 10.1021/bi00425a019. [DOI] [PubMed] [Google Scholar]
- 6.Han XM, Zhou HH. Polymorphism of CYP450 and cancer susceptibility. Acta Pharmacol Sin. 2000;21:673–679. [PubMed] [Google Scholar]
- 7.Snawder JE, Lipscomb JC. Interindividual variance of cytochrome P450 forms in human hepatic microsomes: correlation of individual forms with xenobiotic metabolism and implications in risk assessment. Regul Toxicol Pharmacol. 2000;32:200–209. doi: 10.1006/rtph.2000.1424. [DOI] [PubMed] [Google Scholar]
- 8.Stephens EA, Taylor JA, Kaplan N, Yang CH, Hsieh LL, Lucier GW, Bell DA. Ethnic variation in the CYP2E1 gene: polymorphism analysis of 695 African-Americans, European-Americans and Taiwanese. Pharmacogenetics. 1994;4:185–192. doi: 10.1097/00008571-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe J, Hayashi S, Kawajiri K. Different regulation and expression of the human CYP2E1 gene due to the RsaI polymorphism in the 5'-flanking region. J Biochem. 1994;116:321–326. doi: 10.1093/oxfordjournals.jbchem.a124526. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Hakkola J, Oscarson M, Ingelman-Sundberg M. Structural and functional characterization of the 5'-flanking region of the rat and human cytochrome P450 2E1 genes: identification of a polymorphic repeat in the human gene. Biochem Biophys Res Commun. 1999;263:286–293. doi: 10.1006/bbrc.1999.1362. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi S, Watanabe J, Kawajiri K. Genetic polymorphisms in the 5'-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991;110:559–565. doi: 10.1093/oxfordjournals.jbchem.a123619. [DOI] [PubMed] [Google Scholar]
- 12.Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002;22:185–194. doi: 10.1055/s-2002-30106. [DOI] [PubMed] [Google Scholar]
- 13.Niemelä O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 14.Pirttiaho HI, Salmela PI, Sotaniemi EA, Pelkonen RO, Pitkänen U, Luoma PV. Drug metabolism in diabetic subjects with fatty livers. Br J Clin Pharmacol. 1984;18:895–899. doi: 10.1111/j.1365-2125.1984.tb02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karvonen I, Stengård JH, Huupponen R, Stenbäck FG, Sotaniemi EA. Effects of enzyme induction therapy on glucose and drug metabolism in obese mice model of non-insulin dependent diabetes mellitus. Diabetes Res. 1989;10:85–92. [PubMed] [Google Scholar]
- 16.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 17.Vidali M, Stewart SF, Rolla R, Daly AK, Chen Y, Mottaran E, Jones DE, Leathart JB, Day CP, Albano E. Genetic and epigenetic factors in autoimmune reactions toward cytochrome P4502E1 in alcoholic liver disease. Hepatology. 2003;37:410–419. doi: 10.1053/jhep.2003.50049. [DOI] [PubMed] [Google Scholar]
- 18.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair JF, Szakacs JG, Wood SG, Walton HS, Bement JL, Gonzalez FJ, Jeffery EH, Wrighton SA, Bement WJ, Sinclair PR. Short-term treatment with alcohols causes hepatic steatosis and enhances acetaminophen hepatotoxicity in Cyp2e1 (-/-) mice. Toxicol Appl Pharmacol. 2000;168:114–122. doi: 10.1006/taap.2000.9023. [DOI] [PubMed] [Google Scholar]
- 20.Järveläinen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 21.Agúndez J, Ladero J, Díaz-Rubio M, Benítez J. Rsa I polymorphism at the cytochrome P4502E1 locus is not related to the risk of alcohol-related severe liver disease. Liver. 1996;16:380–383. doi: 10.1111/j.1600-0676.1996.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 22.Donohue TM, Clemens DL, Galli A, Crabb D, Nieto N, Kato J, Barve SS. Use of cultured cells in assessing ethanol toxicity and ethanol-related metabolism. Alcohol Clin Exp Res. 2001;25:87S–93S. doi: 10.1097/00000374-200105051-00016. [DOI] [PubMed] [Google Scholar]
- 23.Kunitoh S, Imaoka S, Hiroi T, Yabusaki Y, Monna T, Funae Y. Acetaldehyde as well as ethanol is metabolized by human CYP2E1. J Pharmacol Exp Ther. 1997;280:527–532. [PubMed] [Google Scholar]
- 24.Ingelman-Sundberg M, Ronis MJ, Lindros KO, Eliasson E, Zhukov A. Ethanol-inducible cytochrome P4502E1: regulation, enzymology and molecular biology. Alcohol Alcohol Suppl. 1994;2:131–139. [PubMed] [Google Scholar]
- 25.Maher J. The CYP2E1 knockout delivers another punch: first ASH, now NASH. Alcoholic steatohepatitis. Nonalcoholic steatohepatitis. Hepatology. 2001;33:311–312. doi: 10.1053/jhep.2001.0330311. [DOI] [PubMed] [Google Scholar]
- 26.Ekström G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 27.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 28.Clot P, Albano E, Eliasson E, Tabone M, Aricò S, Israel Y, Moncada C, Ingelman-Sundberg M. Cytochrome P4502E1 hydroxyethyl radical adducts as the major antigen in autoantibody formation among alcoholics. Gastroenterology. 1996;111:206–216. doi: 10.1053/gast.1996.v111.pm8698201. [DOI] [PubMed] [Google Scholar]
- 29.Albano E, French SW, Ingelman-Sundberg M. Hydroxyethyl radicals in ethanol hepatotoxicity. Front Biosci. 1999;4:D533–D540. doi: 10.2741/albano. [DOI] [PubMed] [Google Scholar]
- 30.Britton RS, Bacon BR. Role of free radicals in liver diseases and hepatic fibrosis. Hepatogastroenterology. 1994;41:343–348. [PubMed] [Google Scholar]
- 31.Iwahashi K, Miyatake R, Suwaki H, Kinoshita H, Ameno K, Ijiri I, Ishikawa Y, Matsuo Y. [Blood ethanol levels and the CTP2E1 C2 allele] Arukoru Kenkyuto Yakubutsu Ison. 1994;29:190–194. [PubMed] [Google Scholar]
- 32.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–208. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- 34.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 35.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 36.Raucy JL, Lasker JM, Kraner JC, Salazar DE, Lieber CS, Corcoran GB. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–280. [PubMed] [Google Scholar]
- 37.Osmundsen H, Bremer J, Pedersen JI. Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys Acta. 1991;1085:141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- 38.Zangar RC, Novak RF. Effects of fatty acids and ketone bodies on cytochromes P450 2B, 4A, and 2E1 expression in primary cultured rat hepatocytes. Arch Biochem Biophys. 1997;337:217–224. doi: 10.1006/abbi.1996.9785. [DOI] [PubMed] [Google Scholar]


