Abstract
AIM: To investigate the patterns of cell proliferation in proximal and distal colons in normal rats and rats with 1,2-dimethylhydrazine (DMH) induced carcinogenesis using the thymidine analogue bromodeoxyuridine.
METHODS: Colonic crypt cell proliferation was immunohistochemically detected using the anti-bromodeoxyuridine Bu20a monoclonal antibody.
RESULTS: Marked regional differences were found in both groups. Total labelling index (LI) and proliferative zone size in both normal (8.65 ± 0.34 vs 7.2 ± 0.45, 27.74 ± 1.07 vs 16.75 ± 1.45) and DMH groups (13.13 ± 0.46 vs 11.55 ± 0.45, 39.60 ± 1.32 vs 35.52 ± 1.58) were significantly higher in distal than in proximal colon (P < 0.05), although the number of cells per proximal crypt was greater (31.45 ± 0.20 vs 34.45 ± 0.39, 42.68 ± 0.53 vs 49.09 ± 0.65, P < 0.0001). Crypt length, total LI and proliferative zone size all increased in both proximal and distal regions of DMH rats compared to normal controls (P < 0.0001). In DMH-treated rat colon a shift of labelled cells to higher crypt cell positions was demonstrated distally whilst a bi-directional shift was evident proximally (P < 0.05).
CONCLUSION: Our results show that changes in cell proliferation patterns, as assessed by bromodeoxyuridine uptake, can act as a reliable intermediate marker of colonic cancer formation. Observed differences between proliferation patterns in distal and proximal colon may be associated with the higher incidence of tumors in the distal colon.
INTRODUCTION
Abnormality in epithelial proliferation is considered to be a characteristic of both human diseases associated with higher risk of colonic cancer and animal colonic cancer models[1-10]. Hyperproliferation of colonic epithelial cells was observed in tritiated thymidine-labelled colorectal specimens from patients with familial polyposis Coli, adenomas and hereditary non-polyposis colon cancer[11-16]. Assessment of colonic crypt cell proliferation, including detection of increased proliferative state and expansion of the proliferative zone is suggested as a putative intermediate marker of colon cancer risk[17-19]. Evidence from animal studies showed that experimental colonic tumors induced by procarcinogen 1,2-dimethylhydrazine (DMH) are of epithelial origin with a similar histology, morphology and anatomy to human colonic neoplasms[20,21]. Furthermore, prior to the development of colonic cancer, DMH injections result in increased colonic crypt cellularity, colonic crypt cell proliferation and colonic crypt proliferative zone[22,23]. This procarcinogen thus provides an adequate model for kinetic and therapeutic studies of the colorectal cancer[24-33]. In the normal rat colon, the location of the stem cells and the direction of colonocyte migration differ between the distal colon and the proximal colon[34]. Interestingly, differences in the incidence, morphology and clinical behaviour of colonic carcinoma have been identified in the proximal and distal colon[35,36]. However, there are no studies which use bromodeoxyuridine (BrdUrd) to compare cell proliferation patterns in the distal and proximal colonic locations of both normal and colonic cancer animals.
BrdUrd is a thymidine analogue which, after incorporation into normal and malignant cells during the S-phase of the cell cycle, can be detected using a monoclonal antibody. BrdUrd immunohistochemistry offers several advantages over thymidine autoradiography. Firstly, because a radioactive precursor is not required, there is less background interference in the tissue sections. Secondly, a clear distinction between the labelled cells and unlabelled cells is provided. Thirdly, the BrdUrd technique is less time-consuming, thus being more suitable for incorporation into routine diagnostic services. Finally, because BrdUrd is also a therapeutic agent, BrdUrd may be safely injected into the human body to study the cell proliferation in the patients without introducing additional procedures[37]. Several studies have demonstrated that the LI estimated by BrdUrd immunohistochemistry is equivalent to that obtained by thymidine autoradiography when an in vitro labelling technique is used[38]. This suggests that BrdUrd LI may be used to replace thymidine LI in both in vitro and in vivo studies.
In the present study, BrdUrd in vivo cell labelling was employed to determine the crypt cell proliferation patterns in the proximal and distal rat colons from normal and DMH-induced colon cancer animals.
MATERIALS AND METHODS
Twelve male Wistar rats with initial weight between 180 g and 220 g were housed, 3 in a cage, and maintained on standard laboratory diet (41 B (M)) with free access to water. Six of these animals received weekly subcutaneous injections of colonic procarcinogen 1,2-dimethylhydrazine (DMH, Aldrich Chemical Company Inc) at a dosage of 20 mg/kg body weight for 20 wk. The DMH was prepared as a 0.5% solution in 1 mM ethylenediaminetetra-acetic acid (EDTA, BDH Limited Poole, England) adjusted to pH7.0 with 10% sodium bicarbonate immediately before injection. Animals were injected at the same time on the same day each week. Animals were sacrificed 2 wk after the last DMH injection. Fifteen minutes before removal of the colon, the anaesthetized rats received a peritoneal injection of 5 mg 2% BrdUrd (Sigma B-5002). This was always done between 9 a.m. and 11 a.m. to avoid diurnal variation. The colon was removed and rinsed with tap water. Following excision of the caecum and rectum, the remaining colon was divided into proximal and distal halves. A 1-2 cm segment of each end of the proximal and distal colon was discarded. After fixation in 70% ethanol for 4 h, the segments were rolled prior to processing and embedding in paraffin wax.
BrdUrd immunohistochemistry
Several 3 μm sections were cut and placed on poly-L-lysine coated slides. The slides were dewaxed before DNA was denatured in 1 M HCl at 37 °C for 12 min. After rinsing in phosphate buffered saline (PBS, pH7.1) the sections were incubated with 30 μL mouse anti-BrdUrd monoclonal antibody (M 744 Dako, Bucks, England) diluted 1:50 in PBS with 0.05% Tween 20 (PBST) with added normal rat serum diluted 1:25 for 60 min at room temperature. After a further rinsing in PBS, the sections were incubated with biotinylated rabbit anti-mouse F (ab')2 antibody (E 413 Dako, Bucks, England) at a dilution of 1:200 in PBST with added rat serum for 30 min at room temperature. Slides were again rinsed in PBS and then incubated with Streptavidin-Biotin Peroxidase complex (K 377 Dako, Bucks, England) for 30 min at room temperature. Finally, the reaction product was visualized using diaminobenzidine hydrochloride (DAB) (Sigma, Dorset, England) primed with 100 μL of 30% H2O2 (diluted 1:20 with distilled water) for approximately 5 min. After DAB was washed off with distilled water, the sections were lightly counterstained in Harris haematoxylin before dehydration and mounting in DPX.
Counting and scoring
Only complete well-orientated longitudinally sectioned crypts which displayed lumen at the top and muscularis mucosae at the base was used for analysis. Comparisons between the following 4 groups were undertaken: distal colon from normal rats, proximal colon from normal rats, distal colon from DMH rats and proximal colon from DMH rats. To facilitate scoring, each crypt was divided at the base into 2 crypt columns (hemicrypts). Starting at the base of the hemicrypt, cells were numbered up to the luminal surface of the colon to determine the number of cells per hemicrypt (CPC) and then divided into 5 equal compartments each containing the same number of cells. The number and the position of BrdUrd-labelled cells in the hemicrypt were recorded. The proliferative zone, which was expressed as a percentage, was obtained by calculating the difference between the highest and lowest labelled cells in each hemicrypt and dividing this Figureure by the total number of cells in the hemicrypt. Labelling index (LI) was determined for the whole hemicrypt, for each compartment and for the proliferative zone, by dividing the number of labelled cells by the total cells and multiplying by 100. Each hemicrypt was then normalised to a notional 100 cell positions. The frequency of BrdUrd positive cells in each of the 100 positions was recorded.
Statistical analysis
All results are presented as the mean ± SEM. Two design variables were generated: (1) DMH, where 1 represents a case where DMH has been administered, 0 represents otherwise; and (2) POSITION, where 1 represents a sample from the proximal colonic crypt, while 0 represents one from distal colonic crypt. Multivariate linear regression analyses were performed using the two design variables as covariates against each of the measured parameters (Table 1). Whenever appropriate a two sided Student's t test was used to identify the differences between individual variables. Kolmogorov-Smirnov 2 sample test (3) was used to compare the BrdUrd cumulative labelling frequency curves in the four subsets in the sample identified with reference to (1) the presence of DMH treatment, and (2) the site of origin of the sample from the colon. Results were considered to be significant when P < 0.05 in all cases.
Table 1.
Multivariate linear regression analyses with 2 design variables against 7 measured parameters
| Response variable | Covariate | Coefficient | P-value |
| CPC | DMH | 12.386 | < 0.05 |
| POSITION | 5.004 | < 0.05 | |
| TOTLI | DMH | 4.433 | < 0.05 |
| POSITION | -1.528 | < 0.05 | |
| INDLI 1 | DMH | 3.915 | < 0.05 |
| POSITION | -9.006 | < 0.05 | |
| INDLI 2 | DMH | 4.504 | < 0.05 |
| POSITION | -2.159 | 0.14 | |
| INDLI 3 | DMH | 10.425 | < 0.05 |
| POSITION | 3.343 | < 0.05 | |
| PZONE | DMH | 18.169 | < 0.05 |
| POSITION | -4.454 | < 0.05 | |
| PZONELI | DMH | -18.312 | < 0.05 |
| POSITION | 1.725 | 0.43 |
CPC, cell per hemicrypt; TOTLI, total hemicrypt labelling index; INDLI 1, individual LI in compartment 1; INDLI 2 individual LI in compartment 2; INDL 3, individual LI in compartment 3; PZONE, proliferative zone; PZONELI, labelling index of proliferative zone.
RESULTS
Proximal compared to distal colon
CPC was significantly greater in the proximal compared to the distal colon in both normal (t = 7.42, P < 0.0001) and DMH-treated groups (t = 7.76, P < 0.0001, Table 1). The total hemicrypt LI was significantly higher in distal than proximal colon in both normal (t = 2.43, P = 0.016) and DMH treated animals (t = 2.47, P = 0.014). In the normal controls, BrdUrd labelled cells in the proximal colon were located predominantly in compartment 2 and 3 (88.4%), whereas the labelled cells in the distal colon were found mostly in compartment 1 and 2 (85.3%). In compartment 1, LI was significantly higher in distal than in proximal colon (t = 8.05, P < 0.0001), while in compartment 3 LI was significantly lower in distal than in proximal colon (t = -4.46, P = 0.0001, Figure 1). Labelled cells never appeared in compartment 5. The size of the proliferative zone was higher distally (t = 2.57, P = 0.011). When the cumulative labelling distribution curves of the proximal and distal colons of normal rats were compared the distal colon showed a significant shift to the left (K-S = 2.192, P < 0.0001, Figure 2).
Figure 1.
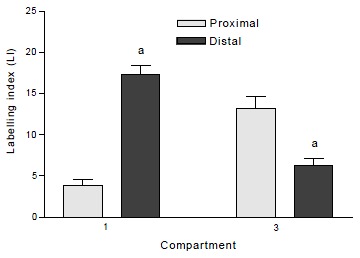
Labelling indices of compartment 1 and compartment 3 in proximal and distal colon of normal rats. aP < 0.0001 when distal is compared to proximal colon in the same compartment. Values are mean ± SEM.
Figure 2.
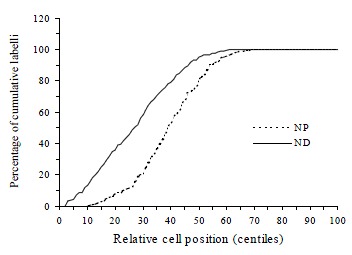
The different patterns of cumulative labelling distributions in proximal (NP) and distal (ND) rat colon of normal controls. The curve is significantly shifted towards the right when the proximal colon is compared to distal colon.
DMH compared with control rats
DMH treatment significantly increased CPC in both proximal (t = 15.9, P < 0.0001) and distal colon (t = 20.6, P < 0.0001) when compared with normal controls. Total LI was also increased significantly by DMH injections in both proximal (t = 6.23, P < 0.0001) and distal colon (t = 7.94, P < 0.0001, Table 2). The effects of DMH on the individual LIs for the 5 compartments in the proximal and distal colon are shown in Figure 3 and Figure 4. In proximal colon, the increase in LI was in compartments 1 and 3, whereas in distal colon, the increase in LI in DMH rats was most marked in compartments 2 and 3. The extent of the increase in LI in compartment 3 of the distal colon was significantly greater than the corresponding increase in compartment 3 LI of the proximal colon (t = 3.44, P = 0.001). Additionally, DMH increased the size of the proliferative zone in both proximal (t = 7.9, P < 0.0001) and distal colonic crypts (t = 10.6, P < 0.0001). However the LI of proliferative zone was reduced because of the increased size. Further analysis of the cumulative labelling distributions showed a shift of the DMH distal curve to the 81st centile which was to the right of the plateau of the normal distal colon located at 61st centile (K-S = 1.89, P = 0.001, Figure 5). In contrast the cumulative labelling distribution curve in proximal colon demonstrated a shift to the left in the lower crypt cell positions and then shifted to the right high up the crypt (K-S = 3.625, P < 0.0001, Figure 6).
Table 2.
Comparison of cell proliferation in proximal and distal hemicrypts of normal and DMH rat colon
| Parameters |
Normal control |
DMH |
||
| Proximal | Distal | Proximal | Distal | |
| Cells/hemi-crypt | 34.45 ± 0.39 | 31.45 ± 0.2a | 49.09 ± 0.65b | 42.68 ± 0.53ac |
| Labelled cells | 2.49 ± 0.16 | 2.74 ± 0.11 | 5.7 ± 0.24b | 5.67 ± 0.23c |
| Total LI | 7.2 ± 0.45 | 8.65 ± 0.34a | 11.55 ± 0.45b | 13.13 ± 0.46ac |
| Proliferative zone | 16.75 ± 1.45 | 21.74 ± 1.07a | 35.52 ± 1.58b | 39.6 ± 1.32ac |
| LI of proliferative zone | 59.43 ± 3.39 | 56.55 ± 2.13 | 39.84 ± 1.77b | 38.89 ± 1.5c |
Values expressed as mean ± SEM.
P < 0.05 when distal is compared to proximal in the same group,
P < 0.05 when DMH proximal is compared to normal proximal,
P < 0.05 when DMH distal compared to normal distal.
Figure 3.
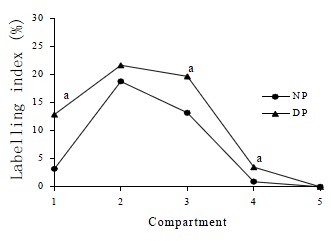
Labelling indices of normal proximal colon (NP) and DMH proximal colon (DP) for the 5 compartments. aP < 0.0001 when labelling indices in the compartments of DMH proximal colons were compared to those found in normal proximal colons in the same compartments.
Figure 4.
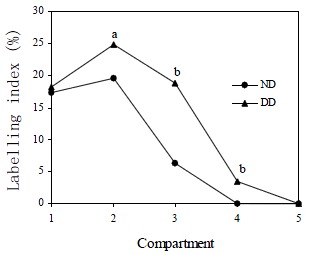
Labelling indices of normal distal colon (ND) and DMH distal colon (DD) for the 5 compartments. aP < 0.05, bP < 0.0001 when labelling indices in the compartments of DMH distal colons were compared to those found in normal distal colons in the same compartments.
Figure 5.
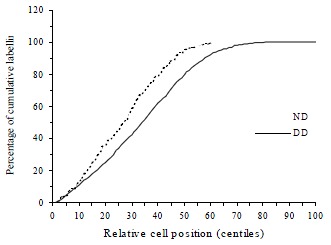
Cumulative labelling distribution in normal (ND) and DMH distal (DD) rat colon. The curve is significantly shifted towards the right in DMH distal colon compared to normal distal colon.
Figure 6.
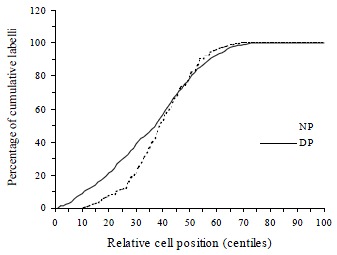
Cumulative labelling distribution in normal (NP) and DMH proximal (DP) rat colon. The curve of DMH treated proximal rat colon is initially significantly shifted towards the left and then in the higher centiles shifted to the right.
DISCUSSION
Significant regional differences in the distribution of BrdUrd-labelled cells located in proximal and distal rat colon have been demonstrated in this study. The total LI and the proliferative zone size are all significantly larger in the distal than in the proximal colonic crypt both in normal and DMH-induced carcinogenesis animals. These regional differences in proliferative cell distribution between proximal and distal colon were previously shown using other markers, e.g. 3H-thymidine autoradiography and proliferating cell nuclear antigen (PCNA) immunohistochemistry. 3H-thymidine LI and proliferative zone size have been reported to be significantly larger distally than proximally[39]. In the distal colon, PCNA expression was strictly confined in the lower third of the crypt, whereas in the proximal colon it was located in the mid-crypt[23].
Our results show that in the distal colon the proliferative cells are located predominantly in the compartments 1 and 2, whereas in the proximal colon, the proliferative cells are located in the 2nd and 3rd compartments. In earlier studies, using tritiated thymidine, Sunter et al[40] found the differences in the distribution of proliferative activity within the crypt from site to site along the length of the rat colon. In the proximal colon, Sunter noted that the peak LI was located in the middle third of the crypt while in distal colon the peak was located in the lower third near the base of the crypt.
These findings together with ours, tend to support the explanation of crypt cell origin and colonocyte migration described by Sato and Ahnen[34]. After double labelling with 3H-thymidine and BrdUrd, they investigated the location of stem cells and the direction of colonocyte migration in normal rat colonic crypt. They reported that distal stem cells are located in the crypt base while proximal stem cells are located in the mid-crypt. They postulated that colonocytes migrate up toward the luminal surface in distal colon in contrast to the bidirectional migration, i.e. up toward the luminal surface and down toward the crypt base in proximal colon.
Our results show that after DMH injection colonic crypt cell proliferation is significantly increased regardless of the position (proximal or distal) and irrespective of the proliferative parameter assessed except the LI of proliferative zone (Table 1). The LI of the proliferative zone in the DMH animals may not increase because of the concomitant increase of the zone size. DMH treatment not only increases the colonic crypt cellularity, total BrdUrd LI but also increases the size of the proliferative zone in both proximal and distal colon. The LI of each compartment from 1 to 3 is also increased, especially in the distal colon. This can be confirmed by the cumulative labelling distribution curves (Figure 5 and Figure 6). Although DMH treatment increases LI in both proximal and distal colon the cumulative labelling distribution is markedly shifted to the right in distal colon whereas the proximal curve shifts to the left (i.e. downwards in the crypt). These results are interesting because of the differences in colonic cancer distribution between proximal and distal colon. The distribution of DMH-induced colorectal cancer resembles human large bowel carcinoma, in which the majority of tumors are recorded distally[41,42]. In our previous work when total colon was exposed to the procarcinogen DMH, 73% tumors occurred distally and only 12% occurred proximally[33].
Further investigation is required to resolve the questions concerning the differences of tumor distribution and their relationship to the different crypt cell proliferation patterns observed in proximal and distal colon. It has been shown that in the proximal colon the lower one-third of the crypt contains predominantly mucous cells whereas the upper one-third mainly has columnar cells[43]. In contrast, crypts of the distal colon contain only a small number of mucous cells in basal positions. The undifferentiated cells or the cells with the lowest level of differentiation (presumptive stem cells) are the vacuolated cells located near or at the crypt base[44]. When the vacuolated cells migrate upward, they transformed into columnar cells and when they migrate downward, they give rise to mucous cells[45]. The major role which stem cells play in carcinogenesis is presumably to transform the malignancy[46]. If this hypothesis is true, there should be more mucinous tumors expected in proximal colon and the tumors in distal colon should originate from columnar cells. In DMH-induced rat colonic carcinogenesis the tumors tended to be more frequently sessile, often mucinous and invasive adenocarcinomas in proximal regions and polyps in distal areas[42].
In this study we observed a greater baseline value of crypt length in proximal than in distal colon, which is contrary to some other published literature[39,40]. This disparity may be due to the differences in defining the criteria for handling the overlapping nuclei, selecting crypts or ascertaining the top of the crypt. We have noticed that the nuclei in the proximal colon are not as typical as those in distal colon. In this study longitudinally well-oriented crypts was selected and all visible nuclei were counted.
Our study demonstrated regional differences in crypt cellularity and cell proliferation patterns. Additionally, we can conclude that the procarcinogen DMH increased crypt cell proliferation and shifted the cumulative BrdUrd labelling distribution in both distal and proximal rat colon. The histochemical similarity of the distal rat colon to the human colon[43] permits the distal rat colon to be used as a model of colonic cancer[47-54]. Therefore, BrdUrd is an appropriate intermediate marker for the early detection of colorectal cancer in patients at high risk and the correct assessment of dietary interference.
ACKNOWLEDGMENTS
We thank Dr. LY Hin for his statistical advice and Dr. PW Hamilton for his critical suggestion.
Footnotes
Edited by Ma JY
Supported in part by DHSS of Northern Ireland.
References
- 1.Chapkin RS, Lupton JR. Colonic cell proliferation and apoptosis in rodent species. Modulation by diet. Adv Exp Med Biol. 1999;470:105–118. doi: 10.1007/978-1-4615-4149-3_12. [DOI] [PubMed] [Google Scholar]
- 2.Wong WM, Wright NA. Cell proliferation in gastrointestinal mucosa. J Clin Pathol. 1999;52:321–333. doi: 10.1136/jcp.52.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills SJ, Mathers JC, Chapman PD, Burn J, Gunn A. Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut. 2001;48:41–46. doi: 10.1136/gut.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu JW, Yu BM, Ji YB, Zheng MH, Li DH. Upregulation of vascular endothelial growth factor by hydrogen peroxide in human colon cancer. World J Gastroenterol. 2002;8:153–157. doi: 10.3748/wjg.v8.i1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng ZH, Xing TH, Qiu GQ, Tang HM. Relationship between Fas/FasL expression and apoptosis of colon adenocarcinoma cell lines. World J Gastroenterol. 2001;7:88–92. doi: 10.3748/wjg.v7.i1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie B, He SW, Wang XD. Effect of gastrin on protein kinase C and its subtype in human colon cancer cell line SW480. World J Gastroenterol. 2000;6:304–306. doi: 10.3748/wjg.v6.i2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochsenkühn T, Bayerdörffer E, Meining A, Schinkel M, Thiede C, Nüssler V, Sackmann M, Hatz R, Neubauer A, Paumgartner G. Colonic mucosal proliferation is related to serum deoxycholic acid levels. Cancer. 1999;85:1664–1669. [PubMed] [Google Scholar]
- 8.Akedo I, Ishikawa H, Ioka T, Kaji I, Narahara H, Ishiguro S, Suzuki T, Otani T. Evaluation of epithelial cell proliferation rate in normal-appearing colonic mucosa as a high-risk marker for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:925–930. [PubMed] [Google Scholar]
- 9.Barnes CJ, Hardman WE, Cameron IL. Presence of well-differentiated distal, but not poorly differentiated proximal, rat colon carcinomas is correlated with increased cell proliferation in and lengthening of colon crypts. Int J Cancer. 1999;80:68–71. doi: 10.1002/(sici)1097-0215(19990105)80:1<68::aid-ijc14>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Kozoni V, Tsioulias G, Shiff S, Rigas B. The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen. Carcinogenesis. 2000;21:999–1005. doi: 10.1093/carcin/21.5.999. [DOI] [PubMed] [Google Scholar]
- 11.Lipkin M, Blattner WE, Fraumeni JF, Lynch HT, Deschner E, Winawer S. Tritiated thymidine (phi p, phi h) labeling distribution as a marker for hereditary predisposition to colon cancer. Cancer Res. 1983;43:1899–1904. [PubMed] [Google Scholar]
- 12.Sun K, Jin BQ, Feng Q, Zhu Y, Yang K, Liu XS, Dong BQ. Identification of CD226 ligand on colo205 cell surface. World J Gastroenterol. 2002;8:108–113. doi: 10.3748/wjg.v8.i1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao B, Jing B, Zhang YL, Zhou DY, Zhang WD. Tumor growth inhibition effect of hIL-6 on colon cancer cells transfected with the target gene by retroviral vector. World J Gastroenterol. 2000;6:89–92. doi: 10.3748/wjg.v6.i1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipkin M, Blattner WA, Gardner EJ, Burt RW, Lynch H, Deschner E, Winawer S, Fraumeni JF. Classification and risk assessment of individuals with familial polyposis, Gardner's syndrome, and familial non-polyposis colon cancer from [3H]thymidine labeling patterns in colonic epithelial cells. Cancer Res. 1984;44:4201–4207. [PubMed] [Google Scholar]
- 15.Wilson RG, Smith AN, Bird CC. Immunohistochemical detection of abnormal cell proliferation in colonic mucosa of subjects with polyps. J Clin Pathol. 1990;43:744–747. doi: 10.1136/jcp.43.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terpstra OT, van Blankenstein M, Dees J, Eilers GA. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987;92:704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Yoshitake K, Sato M, Ahnen DJ. Proliferating cell nuclear antigen expression in normal, preneoplastic, and neoplastic colonic epithelium of the rat. Gastroenterology. 1992;103:160–167. doi: 10.1016/0016-5085(92)91109-h. [DOI] [PubMed] [Google Scholar]
- 18.Colussi C, Fiumicino S, Giuliani A, Rosini S, Musiani P, Macrí C, Potten CS, Crescenzi M, Bignami M. 1,2-Dimethylhydrazine-induced colon carcinoma and lymphoma in msh2 (-/-) mice. J Natl Cancer Inst. 2001;93:1534–1540. doi: 10.1093/jnci/93.20.1534. [DOI] [PubMed] [Google Scholar]
- 19.Anti M, Armuzzi A, Morini S, Iascone E, Pignataro G, Coco C, Lorenzetti R, Paolucci M, Covino M, Gasbarrini A, et al. Severe imbalance of cell proliferation and apoptosis in the left colon and in the rectosigmoid tract in subjects with a history of large adenomas. Gut. 2001;48:238–246. doi: 10.1136/gut.48.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maskens AP. Histogenesis and growth pattern of 1,2-dimethylhydrazine-induced rat colon adenocarcinoma. Cancer Res. 1976;36:1585–1592. [PubMed] [Google Scholar]
- 21.Ma Q, Hoper M, Anderson N, Rowlands BJ. Effect of supplemental L-arginine in a chemical-induced model of colorectal cancer. World J Surg. 1996;20:1087–1091. doi: 10.1007/s002689900165. [DOI] [PubMed] [Google Scholar]
- 22.Richards TC. Early changes in the dynamics of crypt cell populations in mouse colon following administration of 1,2-dimethylhydrazine. Cancer Res. 1977;37:1680–1685. [PubMed] [Google Scholar]
- 23.Heitman DW, Grubbs BG, Heitman TO, Cameron IL. Effects of 1,2-dimethylhydrazine treatment and feeding regimen on rat colonic epithelial cell proliferation. Cancer Res. 1983;43:1153–1162. [PubMed] [Google Scholar]
- 24.Whiteley LO, Klurfeld DM. Are dietary fiber-induced alterations in colonic epithelial cell proliferation predictive of fiber's effect on colon cancer? Nutr Cancer. 2000;36:131–149. doi: 10.1207/S15327914NC3602_1. [DOI] [PubMed] [Google Scholar]
- 25.Cascinu S, Ligi M, Del Ferro E, Foglietti G, Cioccolini P, Staccioli MP, Carnevali A, Luigi Rocchi MB, Alessandroni P, Giordani P, et al. Effects of calcium and vitamin supplementation on colon cell proliferation in colorectal cancer. Cancer Invest. 2000;18:411–416. doi: 10.3109/07357900009032811. [DOI] [PubMed] [Google Scholar]
- 26.Pereira MA. Prevention of colon cancer and modulation of aberrant crypt foci, cell proliferation, and apoptosis by retinoids and NSAIDs. Adv Exp Med Biol. 1999;470:55–63. doi: 10.1007/978-1-4615-4149-3_6. [DOI] [PubMed] [Google Scholar]
- 27.Schmelz EM, Sullards MC, Dillehay DL, Merrill AH. Colonic cell proliferation and aberrant crypt foci formation are inhibited by dairy glycosphingolipids in 1,2-dimethylhydrazine-treated CF1 mice. J Nutr. 2000;130:522–527. doi: 10.1093/jn/130.3.522. [DOI] [PubMed] [Google Scholar]
- 28.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59:5704–5709. [PubMed] [Google Scholar]
- 29.Stammberger P, Baczako K. Cytokeratin 19 expression in human gastrointestinal mucosa during human prenatal development and in gastrointestinal tumours: relation to cell proliferation. Cell Tissue Res. 1999;298:377–381. doi: 10.1007/s004419900085. [DOI] [PubMed] [Google Scholar]
- 30.Walker AR, Segal I. Low-fat dairy foods and colonic epithelial cell proliferation. JAMA. 1999;281:1274. doi: 10.1001/jama.281.14.1274-a. [DOI] [PubMed] [Google Scholar]
- 31.Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–1126. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caderni G, Palli D, Lancioni L, Russo A, Luceri C, Saieva C, Trallori G, Manneschi L, Renai F, Zacchi S, et al. Dietary determinants of colorectal proliferation in the normal mucosa of subjects with previous colon adenomas. Cancer Epidemiol Biomarkers Prev. 1999;8:219–225. [PubMed] [Google Scholar]
- 33.Ma Q, Williamson KE, O'rourke D, Rowlands BJ. The effects of l-arginine on crypt cell hyperproliferation in colorectal cancer. J Surg Res. 1999;81:181–188. doi: 10.1006/jsre.1998.5512. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Ahnen DJ. Regional variability of colonocyte growth and differentiation in the rat. Anat Rec. 1992;233:409–414. doi: 10.1002/ar.1092330308. [DOI] [PubMed] [Google Scholar]
- 35.Freeman HJ, Kim Y, Kim YS. Glycoprotein metabolism in normal proximal and distal rat colon and changes associated with 1,2-dimethylhydrazine-induced colonic neoplasia. Cancer Res. 1978;38:3385–3390. [PubMed] [Google Scholar]
- 36.Lipkin M. Update of preclinical and human studies of calcium and colon cancer prevention. World J Gastroenterol. 1999;5:461–464. doi: 10.3748/wjg.v5.i6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riccardi A, Danova M, Dionigi P, Gaetani P, Cebrelli T, Butti G, Mazzini G, Wilson G. Cell kinetics in leukaemia and solid tumours studied with in vivo bromodeoxyuridine and flow cytometry. Br J Cancer. 1989;59:898–903. doi: 10.1038/bjc.1989.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y, Willems G. Comparison of the classical autoradiographic and the immunohistochemical methods with BrdU for measuring proliferation parameters in colon cancer. Anticancer Res. 1993;13:731–735. [PubMed] [Google Scholar]
- 39.McGarrity TJ, Peiffer LP, Colony PC. Cellular proliferation in proximal and distal rat colon during 1,2-dimethylhydrazine-induced carcinogenesis. Gastroenterology. 1988;95:343–348. doi: 10.1016/0016-5085(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 40.Sunter JP, Watson AJ, Wright NA, Appleton DR. Cell proliferation at different sites along the length of the rat colon. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;32:75–87. doi: 10.1007/BF02889015. [DOI] [PubMed] [Google Scholar]
- 41.Rogers AE, Nauss KM. Rodent models for carcinoma of the colon. Dig Dis Sci. 1985;30:87S–102S. doi: 10.1007/BF01296986. [DOI] [PubMed] [Google Scholar]
- 42.Shamsuddin AK, Trump BF. Colon epithelium. II. In vivo studies of colon carcinogenesis. Light microscopic, histochemical, and ultrastructural studies of histogenesis of azoxymethane-induced colon carcinomas in Fischer 344 rats. J Natl Cancer Inst. 1981;66:389–401. [PubMed] [Google Scholar]
- 43.Shamsuddin AK, Trump BF. Colon epithelium. I. Light microscopic, histochemical, and ultrastructural features of normal colon epithelium of male Fischer 344 rats. J Natl Cancer Inst. 1981;66:375–388. [PubMed] [Google Scholar]
- 44.Nabeyama A. Presence of cells combining features of two different cell types in the colonic crypts and pyloric glands of the mouse. Am J Anat. 1975;142:471–483. doi: 10.1002/aja.1001420405. [DOI] [PubMed] [Google Scholar]
- 45.Chang WW, Leblond CP. Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am J Anat. 1971;131:73–99. doi: 10.1002/aja.1001310105. [DOI] [PubMed] [Google Scholar]
- 46.Pierce GB. Neoplasms, differentiations and mutations. Am J Pathol. 1974;77:103–118. [PMC free article] [PubMed] [Google Scholar]
- 47.Dashwood RH, Xu M, Orner GA, Horio DT. Colonic cell proliferation, apoptosis and aberrant crypt foci development in rats given 2-amino-3-methylimidaz. Eur J Cancer Prev. 2001;10:139–145. doi: 10.1097/00008469-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Holt PR, Arber N, Halmos B, Forde K, Kissileff H, McGlynn KA, Moss SF, Kurihara N, Fan K, Yang K, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11:113–119. [PubMed] [Google Scholar]
- 49.Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin (17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307–313. doi: 10.1002/ijc.1483. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, Uesaka T, Watanabe H, Kato N. High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int J Oncol. 2001;19:1009–1014. doi: 10.3892/ijo.19.5.1009. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z, Tomotake H, Wan G, Watanabe H, Kato N. Combined effect of dietary calcium and iron on colonic aberrant crypt foci, cell proliferation and apoptosis, and fecal bile acids in 1,2-dimethylhydrazine-treated rats. Oncol Rep. 2001;8:893–897. doi: 10.3892/or.8.4.893. [DOI] [PubMed] [Google Scholar]
- 52.Exon JH, South EH, Magnuson BA, Hendrix K. Effects of indole-3-carbinol on immune responses, aberrant crypt foci, and colonic crypt cell proliferation in rats. J Toxicol Environ Health A. 2001;62:561–573. doi: 10.1080/152873901300007842. [DOI] [PubMed] [Google Scholar]
- 53.Jenab M, Thompson LU. Phytic acid in wheat bran affects colon morphology, cell differentiation and apoptosis. Carcinogenesis. 2000;21:1547–1552. [PubMed] [Google Scholar]
- 54.Davidson LA, Brown RE, Chang WC, Morris JS, Wang N, Carroll RJ, Turner ND, Lupton JR, Chapkin RS. Morphodensitometric analysis of protein kinase C beta (II) expression in rat colon: modulation by diet and relation to in situ cell proliferation and apoptosis. Carcinogenesis. 2000;21:1513–1519. [PubMed] [Google Scholar]


