Abstract
AIM: To study the changes of cell proliferation and apoptosis in rat jejunal epithelium at different ages.
METHODS: Cell proliferation and apoptosis of the jejunal mucosal and glandulous epithelia from birth to postnatal 12th month were observed using immunocytochemistry (ICC), and TUNEL method. The height of villus, the thickness of muscle layer and the number of goblet cells in jejunal mucosal and glandulous epithelia were measured by BeiHang analytic software and analyzed by STAT.
RESULTS: (1) Proliferating cell nuclear antigen (PCNA) positive cells of jejunal glandulous recess were found and increased in number from birth to the postnatal 3rd month. The number of PCNA positive cells peaked in the postnatal 3rd month, and decreased from then on. (2) The number of apoptotic cells also peaked in the postnatal 3rd month, showing a similar trend to that of the PCNA positive cells. (3) The height of jejunal villus increased after birth, peaked in the postnatal 3rd month and decreased from then on. The jejunal muscle layer became thicker in the postnatal 3rd week and the postnatal 12th month. The number of goblet cells of the jejunal mucosal and glandulous epithelia had a linear correlation with age.
CONCLUSION: (1) PCNA positive cells are distributed in the jejunal glandulous recess. (2) Apoptotic cell number peaks in the postnatal 3rd month, indicating that cell proliferation and apoptosis are developed with the formation of digestive metabolism as rat grows to maturity. (3) The thickness of jejunal muscle layer increases to a maximum in the postnatal 3rd week, which may be related to the change in diet from milk to solid food. (4) The number of goblet cells increases rapidly in the postnatal 3rd week, probably due to ingestion of solid food.
INTRODUCTION
The small intestine is the primary digestive apparatus of mammals, and nutrient absorption is ongoing mostly via intestinal epithelium. Mathan et al[1-4] in 1976 observed the embryogenesis and postnatal change of rat duodenal villi using transmission electron microscope and scanning electron microscope respectively. Weinstein et al[5-7] described the ultrastructure and function of intestinal epithelial lining in 1981. In 1995, Gao et al[8-10] studied the epithelialization, expression pattern, and transformation of some enzymes of duodenum with histological methods. However, the status of proliferation and apoptotic changes in developing jejunal epithelial lining has not yet been elucidated. The present study was to provide a digestive physiological proof more directly in proliferation and apoptotic changes at four representative developmental stages in rats: birth, postnatal 3rd week, postnatal 3rd month, and postnatal 12th month.
MATERIALS AND METHODS
Materials
Sprague-Dawly rats (male, Grade II, Certificate No 04036, obtained from the Experimental Animal Center of Hebei Province) were divided into four groups: newborn (n = 6), postnatal 3rd week (n = 6), postnatal 3rd month (n = 6) and postnatal 12th month (n = 6). The rats were held for 12 h without food. The mean weight was 5.6 ± 0.3 g for newborn, 53.5 ± 1.2 g for postnatal 3rd week, 390 ± 0.9 g for postnatal 3rd month and 601.7 ± 3.4 g for postnatal 12th month. The abdominal cavity was opened immediately after the rat was killed with ether. Several segments of jejunum (1-2 cm) were removed and placed immediately in a fixative consisting of 4% paraformaldehyde and 0.01 mol/L phosphate buffered saline (PBS, pH7.4) (4 °C, 12 h). The segments were used for TdT-mediated X-dUTP nick end labeling (TUNEL) examination and HE staining, and other segments were placed in a fixative consisting of Bouin’s solution. Fixed jejunum segments were embedded in paraffin and continuously sliced up at 6 μm thickness and mounted onto glass slides covered with 3-aminopropyl-triethoxysilane (APES). They were dried at 37 °C, and used for immunostaining of proliferating cell nuclear antigen (PCNA).
Reagents
Rabbit anti-rat PCNA antibody and SP kit were purchased from Beijing Zhongshan Biotechnology Company. TUNEL examination kit was purchased from Wuhan Boster Biological Technology Company.
HE staining
Several segments of jejunum were fixed in formalin and embedded in paraffin. Slides were cut and stained with hematoxylin and eosin according to routine methods.
Immunohistochemistry
Immunohistochemical staining for PCNA was performed using SP technique with following procedures. Mounted specimens were washed in 0.01 mol/L phosphate-buffered saline (PBS). Endogenous peroxidase was blocked by 0.3% H2O2 in methanol for 25 min. Slides were washed with PBS followed by incubation in normal goat serum for 30 min at room temperature. The primary rabbit anti-rat PCNA antibody was diluted 1:75 and applied to sections for 12 h at 4 °C. Slides were washed with PBS again and incubated with biotinylated secondary antibody for 60 min at 37 °C. After rinsed in PBS, the slides were incubated with peroxidase-conjugated streptavidin for 60 min at 37 °C. Colour reaction was performed by incubating the sections with diaminobenzidine-H2O2 for 5 min. The sections were counterstained with hematoxylin, dehydrated in gradient alcohol, and hyalinized in dimethylbenzene. Finally, the sections were mounted and observed under a microscope. PBS was used as a substitute for primary antibody as negative control.
TdT-mediated X-dUTP nick end labeling (TUNEL) examination technique
Mounted specimens were dewaxed and endogenous peroxidase was blocked by 3% H2O2 in methanol for 10 min. After washed with water, 20 μl labeling buffer (1 μl TdT, 1 μl DIG-UTP, and 18 μl Labeling Buffer) was added to each section and incubated for 12 h at 4 °C. The sections were washed with 0.01 mol/L TBS and 50 μl blocking solution was added to each slide, followed by incubation for 30 min at room temperature. Biotinylated antidigoxin antibody was added to the slides for 30 min at 37 °C. SABC was added for 40 min at 37 °C followed by washing with TBS. Then the specimens were incubated with diaminobenzidine-H2O2 for 5 min. After rinsed in tap water, the sections were counterstained with hematoxylin. Then they were dehydrated in gradient alcohol and hyalinized in dimethylbenzene. The sections were mounted and observed under a microscope. PBS was used as a substitute for primary antibody as negative control.
Data processing
The Northern image analyzing software was employed to analyze the stained area (the percentage of immunoreaction positive cells in total number of cells within a vision field). Three sections were selected from each experimental animal, and ten fields of vision from each section were inspected by statistical random sampling. The villus height was measured, the apoptotic cells under 16 × 40 double OLYMPUS microscope with testing resection and the number of goblet cells in unit area were counted[11]. The proliferation index was calculated. Under 15 × 10 double microscope, the thickness of muscle layer was measured according to the statistical random sampling with a micrometer, then the mean value was calculated. The values were expressed as x ± s. Statistical analysis was performed using the STAT software and analysis of variance was used as appropriate. P < 0.05 was considered significant.
RESULTS
The number of jejunal mucosal goblet cells increased from birth to postnatal 3rd week and decreased from postnatal 3rd week to postnatal 3rd month, and then increased again after the postnatal 3rd month (Figure 1, Table 1). The number of glandulous epithelial goblet cells had a linear correlation with age (Figure 1), and there were significant differences between groups (P < 0.01, Table 1). The height of jejunal villus increased from birth, peaked in the postnatal 3rd month and decreased from then on, and the villi were swollen in postnatal 12th month (Figure 1, Table 1). The thickness of jejunal muscle layer appeared crest on postnatal 3rd week and postnatal 12th month, the muscle fibers were quite dense in the postnatal 3rd month but very loose in the postnatal 3rd week (Table 1).
Figure 1.
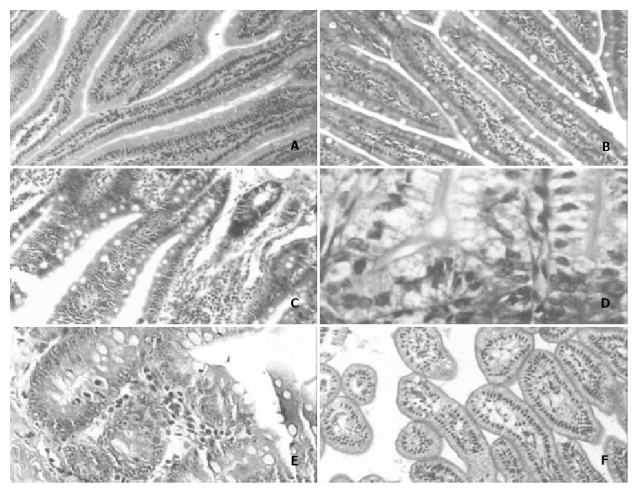
A: The number of goblet cells and the morphology of jejunal villi in the postnatal 3rd month × 100. B: The number of goblet cells in rat jejunal villi in the postnatal 3rd month × 100. C: The number of goblet cells and the morphology of jejunal villi in the postnatal 12th month × 100. D: There were few or no goblet cells in the jejunal glands of newborn rats × 400; E: The number of goblet cells in rat jejunal gland was much higher in the postnatal 12th month than in any other month × 400. F: The jejunal villi of newborn rats displayed ateliosis and were very small × 200.
Table 1.
Developmental changes in cell type and morphology of rat villi after staining (-x ± s, n = 6)
| Group | Number of villus goblet cells (entries/μm2) | Number of crypt goblet cells (entries/μm2) | Thickness of muscle layer (μm) | Villus height (μm) |
| Newborn group | 4.89 ± 2.67 | 0 ± 0 | 32.0 ± 15.71 | 315.20 ± 43.74 |
| Postnatal 3rd week | 7.14 ± 1.54b | 4.47 ± 1.10b | 64.86 ± 14.91b | 532.75 ± 53.36b |
| Postnatal 3rd month | 4.44 ± 1.11 | 5.27 ± 0.93 | 56.34 ± 15.41 | 629.64 ± 48.22 |
| Postnatal 1 year | 9.31 ± 1.50d | 6.49 ± 1.98 | 60.48 ± 11.93 | 510.95 ± 82.93d |
P < 0.01, vs Newborn group.
P < 0.01, vs Postnatal 3rd month.
The immunostaining of PCNA positive cells was shown as light brown deposited in the nuclei. The PCNA positive cells in rat jejunum were mainly distributed in jejunal glandulous recess and proper lamina (Figure 2). The PCNA positive cells were shown to increase from birth to postnatal 3rd month, and decrease from then on (Figure 2, Table 2).
Figure 2.
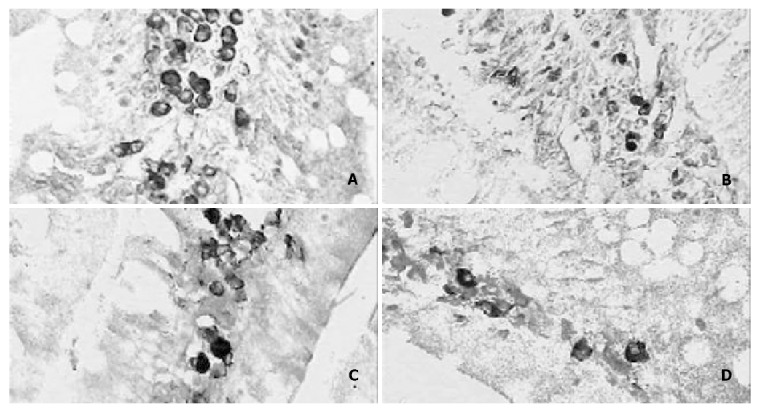
A: The expression of PCNA positive cells of the rat jejunum in the postnatal 3rd month × 400. B: The number of jejunal PCNA positive cells in the postnatal 12th month × 400. C: The expression of apoptotic positive cells of rat jejunum in the postnatal 3rd month × 400. D: The number of rat jejunal apoptotic positive cells in the postnatal 12th month × 400.
Table 2.
Surface densities of PCNA-positive cells and the number of apoptotic cells at different stages (-x ± s, n = 6)
| Age | Surface densities of PCNA-positive cells | The number of apoptotic cells |
| Newborn group | 0 ± 0 | 0 ± 0 |
| Postnatal 3rd week | 0.022 ± 0.012 | 9.71 ± 2.43 |
| Postnatal 3rd month | 0.449 ± 0.063b | 38.83 ± 7.41b |
| Postnatal 1 year | 0.096 ± 0.045d | 11.36 ± 3.14d |
P < 0.01, vs Postnatal 3rd week.
P < 0.01, vs Postnatal 3rd month.
The apoptotic cells were stained by TUNEL and positive cells were shown as dark brown in the nuclei. The apoptotic positive cells were mainly distributed in lamina propria (Figure 2). The number of apoptotic positive cells increased from newborn to postnatal 3rd month, and then decreased with aging. The pattern was similar to that of the PCNA positive cells (Figure 2, Table 2).
DISCUSSION
The goblet cell is a typical cell capable of excreting glycoprotein, containing a lot of spumous grume and granules of inhomogeneous electronic density. The jejunal glandulae of newborn rats could not develop but goblet cells can be seen in the villi. The number of goblet cells increased in the postnatal 3rd month, which might be related to the change in diet from milk to solid food. In the postnatal 12th month, the number of goblet cells increased again, and in this phase the increase of goblet cells led to the increase of mucus, which has the functions of lubricating mucosa and defending intestinal wall. The increasing change in the number of gerontic goblet cells might be a kind of protective mechanism against the weakening of excreting function of digestive gland in gerontic rat.
Cheng et al[12] considered that rat intestinal villi had perfectly developed on the 21st day of pregnancy, but there were only a few intestinal glands and few goblet cells. Our result was coincident with that of Cheng et al. We found that along with the development of intestinal glands the number of goblet cells in the intestinal gland gradually increased in the rat of postnatal 3rd week, postnatal 3rd month and postnatal 12th month, and this phenomenon might be corresponding to hearty digestive function in mature rats.
With the commence of sucking activity of newborn rat, the digestive tract starts its digestive activities. The newborn rat villi are very fine, and immature. In this period the intestinal digestive function is quite feeble, great molecule materials are assimilated via endocytosis with the help of lysosomes, thus the nutrients are absorbed. This absorptive manner only suits to the materials in milk[13]. Milk has balanced and all-sided nutrition, and contains many growth factors which are necessary to the development of the rat, so it can promote intestinal cells to develop[14]. To absorb various kinds of nutrients, gastrointestinal tract of rat develops rapidly and matures gradually to adapt to the digestive function[15]. In the postnatal 3rd week, villi become primarily matured. The muscle layer is quite thick, and the enteric digestive function of rat is boosted up. In this phase, endocytosis has weakened, and the rat can gradually digest food, and many absorptive mechanisms like glucide simple diffusing, solvent dragging, active ion transport and co-transport appear. In the postnatal 3rd month, villi heighten and peak, which increase the intestinal digestive areas. In this period, microvilli on the top of intestinal villus can be seen under an electron microscope, and they gather together to be known as brush border. Villi and microvilli increase the intestinal digestive area to 200 m2 or more. There are capillary, lymphatic capillary, smooth muscle fiber and nerve net in intestinal villi, and one or more central small arteries and a central chyle vessel in each intestinal villus. The villus height increases with advancing age. The structures of capillary, lymphatic capillary, smooth muscle fiber and nerve net in intestinal villi are perfectly developed, in favor of substance countercurrent exchange in ascending and descending vessels of villi. At the same time, the muscle fiber relaxation and contraction and the pump function of central chyle vessels in villi help digestion. Besides, increase of muscle fibers which enhance the whip and rhythmic condensing movement is also helpful to digestion, and this period becomes the strongest phase of the digestive function. In the postnatal 12th month, there are effete changes like swelling and shortening of some villi, and the enteric digestive function slacks up, thus food is insufficiently digested in the enterocoelic cavity. The phenomenon of exocytosis manner assimilating large molecule substances appear again, maybe this manner is a kind of compensation for the digestion.
PCNA has a molecular weight of 36 kDa and is an assistant protein of DNA polymerase δ in terms of its physiological function. It can objectively reflect the proliferation degree of cells, and is a better proliferating marker of cells. PCNA immunostaining signal is almost completely disappeared in the nuclei. Positive substance takes on the form of dispersion or granule, or a combination of the two forms. The cell nuclei of strong positive cells are brown-yellow, nuclei of positive cells are yellow, the cell nuclei of feeble positive cells are buff. The proliferating bloom region of PCNA positive cells is distributed in jejunal glandulous recess, and the differentiation of positive cells on the top of villi is a sort of “ladder-like” movement[16-19]. The experiment showed that the proliferative index of PCNA positive cells in the recess increased from birth to postnatal 3rd month, and reached the crest in postnatal 3rd month, which made the cells in recess to be continuously hyperplastic. The proliferation index decreased at postnatal 12th month, indicating that the proliferation of cells becomes weak as the organism ages.
As the fastest renewing cells in the body, the cell cycle of small intestinal epithelia only exists for 12 h, and they also have apoptosis[20-23]. As early as the end of 19th century, the granules of epithelial cells in embryonic digestive tube under optical microscope were named “meconium corpuscles”. Harmon et al[24-27] confirmed under the electron microscope that in fact meconium corpuscles were the apoptotic globules in digestive tract epithelia. Iwanaga et al[17,28-33] considered that macrophages in small intestinal proper lamina of adult rats could induce apoptosis of epithelia. It was observed in this experiment that the apoptotic cell number of intact jejunal villi of SD rats increased from birth to postnatal 3rd month and reached its peak in postnatal 3rd month, which may be indicated that when the rat grows up, cell proliferation and apoptosis activity became abundant with the buildup of body metabolism function. The metabolic level decreased in postnatal 12th month with the senescence of body, the number of apoptotic cells began to decline compared with that in the postnatal 3rd month.
Footnotes
Supported by the Natural Science Foundation of Hebei Province, No. 303158; Education Department Foundation of Hebei Province, No. 2002136
Edited by Zhu LH and Wang XL
References
- 1.Mathan M, Moxey PC, Trier JS. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976;146:73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- 2.Ono K. Changes of the caecal villi during postnatal development in rats. Cell Tissue Res. 1980;208:253–259. doi: 10.1007/BF00234875. [DOI] [PubMed] [Google Scholar]
- 3.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 4.Varedi M, Greeley GH, Herndon DN, Englander EW. A thermal injury-induced circulating factor(s) compromises intestinal cell morphology, proliferation, and migration. Am J Physiol. 1999;277:G175–G182. doi: 10.1152/ajpgi.1999.277.1.G175. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein L, Edelstein SM, Madara JL, Falchuk KR, McManus BM, Trier JS. Intestinal cryptosporidiosis complicated by disseminated cytomegalovirus infection. Gastroenterology. 1981;81:584–591. [PubMed] [Google Scholar]
- 6.Pácha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80:1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- 7.Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Gao JG, Wang SP, Xing SG. Histological and histochemical ob-servation on the development and differentiation of the mouse duodenum. Jiepao Xuebao. 1995;26:425–430. [Google Scholar]
- 9.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 10.Plateroti M, Freund JN, Leberquier C, Kedinger M. Mesenchyme-mediated effects of retinoic acid during rat intestinal development. J Cell Sci. 1997;110(Pt 10):1227–1238. doi: 10.1242/jcs.110.10.1227. [DOI] [PubMed] [Google Scholar]
- 11.Zheng FS. The cell morphometry. Beijing: Beijing Medical University and PUMC United Publishing House. 1990:11. [Google Scholar]
- 12.Cheng D, Chen AJ, Yin X, Xu YL, Zhu XX. Morphogenesis of Duodenal Villi in Rat. Jiepao Xuebao. 1990;21:437–440. [Google Scholar]
- 13.Cheng LZ. Histology. 2nd Ed. Beijing: The Peoples’ Health Publishing House. 1993:1106. [Google Scholar]
- 14.Li ZB. Research progress in gut development of newborn. Guowai Yixue Er Kexue Fence. 2000;27:9–23. [Google Scholar]
- 15.Mubiru JN, Xu RJ. Growth and development of the exocrine pancreas in newborn pigs: the effect of colostrum feeding. Biol Neonate. 1997;71:317–326. doi: 10.1159/000244431. [DOI] [PubMed] [Google Scholar]
- 16.Xie JX, Gu Y, Zhao SM, Luo BG, Chen LL, Wu ZH, Zuo HT. Study on changes of apoptosis and expression of PCNA in atrophic intestinal mucosal epithelia. Jiepaoxue Zazhi. 1999;22:124–127. [Google Scholar]
- 17.Varedi M, Chinery R, Greeley GH, Herndon DN, Englander EW. Thermal injury effects on intestinal crypt cell proliferation and death are cell position dependent. Am J Physiol Gastrointest Liver Physiol. 2001;280:G157–G163. doi: 10.1152/ajpgi.2001.280.1.G157. [DOI] [PubMed] [Google Scholar]
- 18.Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- 19.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 20.Iiboshi Y, Nezu R, Kennedy M, Fujii M, Wasa M, Fukuzawa M, Kamata S, Takagi Y, Okada A. Total parenteral nutrition decreases luminal mucous gel and increases permeability of small intestine. JPEN J Parenter Enteral Nutr. 1994;18:346–350. doi: 10.1177/014860719401800412. [DOI] [PubMed] [Google Scholar]
- 21.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough. Am J Clin Nutr. 2000;71:1603–1610. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 22.Remillard RL, Dudgeon DL, Yardley JH. Atrophied small intestinal responses of piglets to oral feedings of milk. J Nutr. 1998;128:2727S–2729S. doi: 10.1093/jn/128.12.2727S. [DOI] [PubMed] [Google Scholar]
- 23.Jeppesen PB, Mortensen PB. Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut. 2000;46:701–706. doi: 10.1136/gut.46.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmon B, Bell L, Williams L. An ultrastructural study on the "meconium corpuscles" in rat foetal intestinal epithelium with particular reference to apoptosis. Anat Embryol (Berl) 1984;169:119–124. doi: 10.1007/BF00303140. [DOI] [PubMed] [Google Scholar]
- 25.Jordinson M, Goodlad RA, Brynes A, Bliss P, Ghatei MA, Bloom SR, Fitzgerald A, Grant G, Bardocz S, Pusztai A, et al. Gastrointestinal responses to a panel of lectins in rats maintained on total parenteral nutrition. Am J Physiol. 1999;276:G1235–G1242. doi: 10.1152/ajpgi.1999.276.5.G1235. [DOI] [PubMed] [Google Scholar]
- 26.Williams CL, Bihm CC, Rosenfeld GC, Burks TF. Morphine tolerance and dependence in the rat intestine in vivo. J Pharmacol Exp Ther. 1997;280:656–663. [PubMed] [Google Scholar]
- 27.Haertel-Wiesmann M, Liang Y, Fantl WJ, Williams LT. Regulation of cyclooxygenase-2 and periostin by Wnt-3 in mouse mammary epithelial cells. J Biol Chem. 2000;275:32046–32051. doi: 10.1074/jbc.M000074200. [DOI] [PubMed] [Google Scholar]
- 28.Iwanaga T, Hoshi O, Han H, Takahashi-Iwanaga H, Uchiyama Y, Fujita T. Lamina propria macrophages involved in cell death (apoptosis) of enterocytes in the small intestine of rats. Arch Histol Cytol. 1994;57:267–276. doi: 10.1679/aohc.57.267. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto A, Tatsumi H, Maruyama M, Uchiyama T, Okada N, Fujita T. Modulation of intestinal permeability by nitric oxide donors: implications in intestinal delivery of poorly absorbable drugs. J Pharmacol Exp Ther. 2001;296:84–90. [PubMed] [Google Scholar]
- 30.Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol. 1998;274:G270–G276. doi: 10.1152/ajpgi.1998.274.2.G270. [DOI] [PubMed] [Google Scholar]
- 31.Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530–537. doi: 10.1136/gut.42.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, Opas M, Bleackley RC, Green DR, Michalak M. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]


