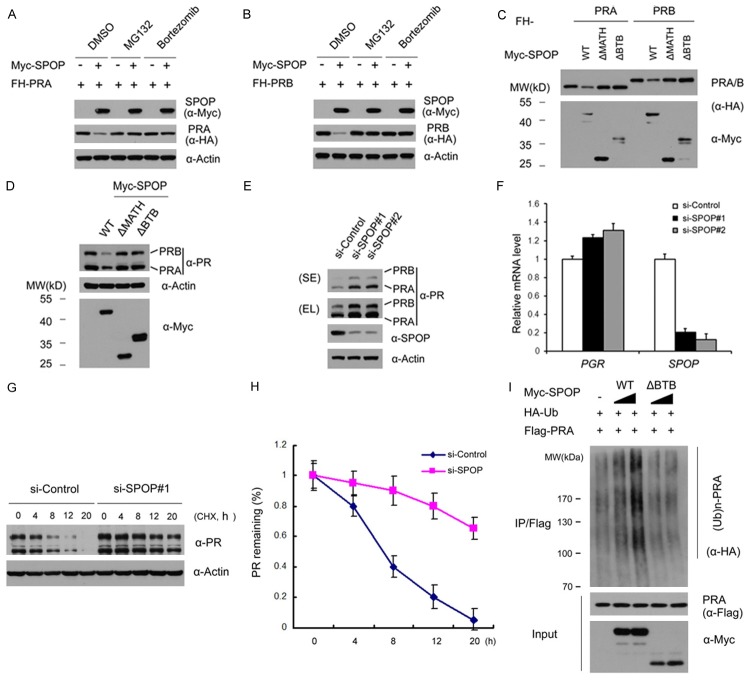
Figure 2.
SPOP targets PR for ubiquitination and degradation. (A, B) 293T cells were transfected with FH-PRA (A) or PRB (B) in combination with or without Myc-SPOP constructs. After 24 hr, cells were treated with MG132 (20 µM), Bortezomib (200 nM), or DMSO for 4 hr before cell lysates were prepared for WB analyses; (C) FH-PRA/B and different amounts of Myc-SPOP-WT or deletion mutants (ΔMATH, ΔBTB) constructs were transfected into 293T cells. After 24 hr, cell lysates were prepared for WB analyses; (D) T47D cells were transfected with Myc-SPOP-WT, or deletion mutants (ΔMATH, ΔBTB) constructs. After 36 hr, cell lysates were prepared for WB analyses; (E) T47D cells were transfected with control siRNAs or two SPOP-specific siRNAs. After 48 hr, cell lysates were prepared for WB analyses; (F) Quantitative RT-PCR measurement of the mRNA levels of SPOP and PGR in SPOP-depleted T47D cells. The mRNA level of GAPDH was used for normalization. The mean values (S.D.) of three independent experiments are shown. (G, H) T47D cells were transfected with control or SPOP-specific siRNAs. After 48 hr, cells were chased with 30 μM cycloheximide (CHX). At the indicated time points, cell lysates were prepared for WB analyses; (G) At each time point, the intensity of PR was first normalized to the intensity of Actin (loading control) and then to the value of the 0-hr time point; (H) The mean values (S.D.) of three independent experiments are shown; (I) SPOP promotes PRA polyubiquitination in vivo. Flag-PRA, HA-Ub and Myc-SPOP-WT or ΔBTB mutant constructs were co-transfected into 293T cells. After 24 hr, cells were treated with 20 µM MG132 for 6 hr. PRA proteins were immunoprecipitated with anti-Flag antibody and resolved by SDS/PAGE. The ubiquitinated forms of PRA were analyzed by WB with anti-HA antibody.