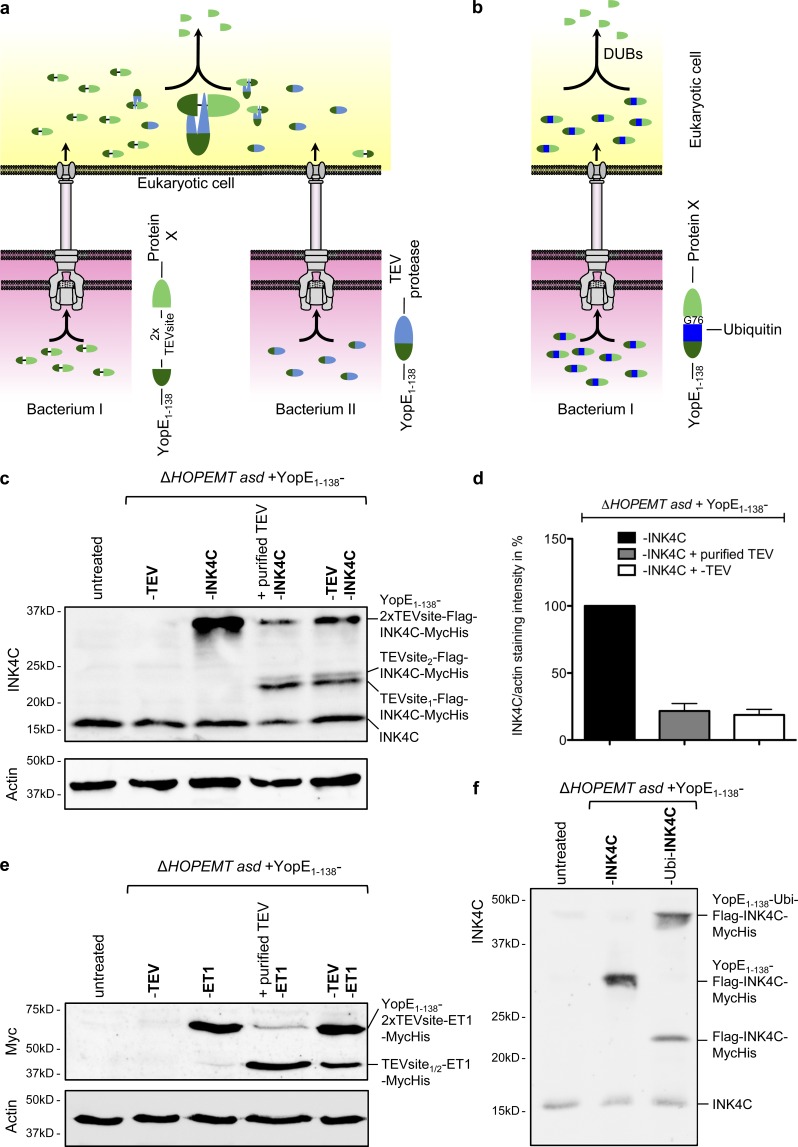
Figure 7.
Cleavage of the YopE1–138 fragment from the translocated fusion protein. (a) Schematic representation of the strategy developed to cleave off the YopE1–138-fragment after T3S-dependent protein delivery into HeLa cells. HeLa cells are coinfected with two different Y. enterocolitica strains. One strain delivers the TEV protease fused to YopE1–138, whereas the other strain delivers a protein of interest fused to YopE1–138 via a linker containing a double TEV protease cleavage site. After protein delivery into the eukaryotic cell, TEV cleaves off the YopE1–138 fragment from the protein of interest X. (b) Schematic representation of the strategy developed to cleave off the YopE1–138 fragment after T3S-dependent protein delivery into HeLa cells by fusion to ubiquitin. Ubiquitin is processed at its C terminus (after G76) by a group of endogenous ubiquitin-specific C-terminal proteases (DUBs), leading to the liberation of the protein C-terminally fused to ubiquitin. (c) TEV-mediated cleavage of the YopE1–138 fragment from the INK4C fusion protein. Digitonin-lysed HeLa cells infected for 2 h with the indicated strains each at an MOI of 100 were analyzed by Western blot with an anti-INK4C antibody for the presence of YopE1–138-2xTEVsite–Flag–INK4C–MycHis or its cleaved form Flag–INK4C–MycHis. In lane 4, cell lysate was incubated overnight with purified TEV protease. (d) Quantification of TEV-mediated cleavage. Cleavage was measured by quantifying the band corresponding to YopE1–138–2x TEV site–Flag–INK4C from panel c normalized to the actin staining intensity. Intensity of the lane 3 band was set to 100%. Data correspond to the mean of n = 2 independent experiments, and error bars indicate the standard error of the mean. (e) TEV-mediated cleavage of the YopE1–138 fragment from the ET1-Myc fusion protein. Digitonin-lysed HeLa cells infected for 1 h with the indicated strains at an MOI of 100 were analyzed by Western blotting with an anti-Myc antibody for the presence of YopE1–138–2x TEV site–ET1–Myc or its cleaved form ET1-Myc. In lane 4, cell lysate was incubated overnight with purified TEV protease. (f) Cleavage of YopE1–138–ubiquitin–Flag–INK4C–MycHis after translocation into HeLa cells. Digitonin-lysed HeLa cells infected for 1 h with the indicated strains each at an MOI of 100 were analyzed by Western blot with an anti-INK4C antibody for the presence of YopE1–138–ubiquitin–Flag–INK4C–MycHis or its cleaved form Flag–INK4C–MycHis.