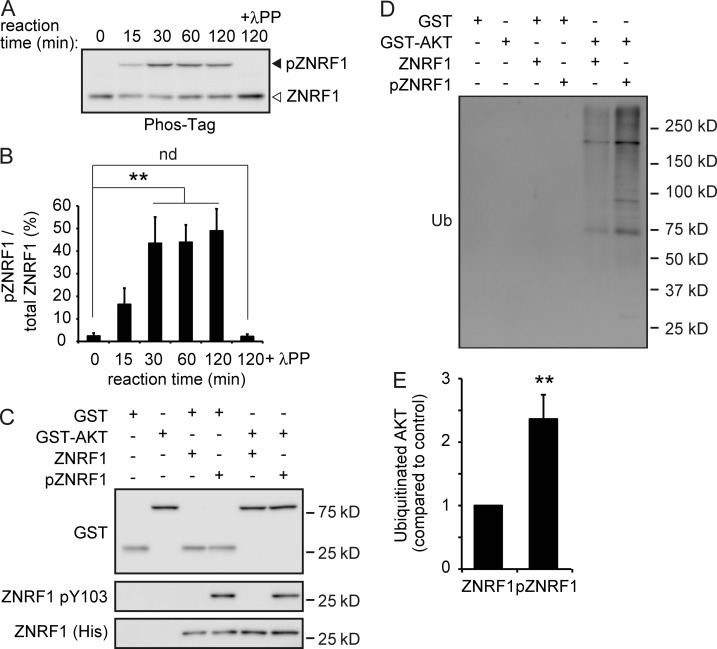
Figure 4.
ZNRF1 is directly phosphorylated by EGFR in vitro. (A and B) The His-ZNRF1 protein was incubated in the presence or absence of a recombinant active EGFR protein in vitro. The mixtures were subjected to Phos-Tag PAGE and analyzed by immunoblotting using an anti-His antibody (A). Quantified levels for phosphorylated ZNRF1 (pZNRF1) relative to total ZNRF1 are shown (B). (C–E) EGFR-dependent ZNRF1 pY103 leads to increased ZNRF1 activity to ubiquitinate AKT in vitro. In vitro ubiquitination assays of GST or GST-AKT were performed using His-ZNRF1 or His-pZNRF1 together with E1, E2, and ubiquitin. Representative immunoblots (C and D) and quantified levels for polyubiquitinated AKT with pZNRF1 relative to the control with ZNRF1 (E) are shown. Data are presented as the mean ± SEM. n = 5. Significant differences from the control (**, P < 0.01) were determined by two-tailed Student’s t test. nd, no significant difference; Ub, ubiquitin.