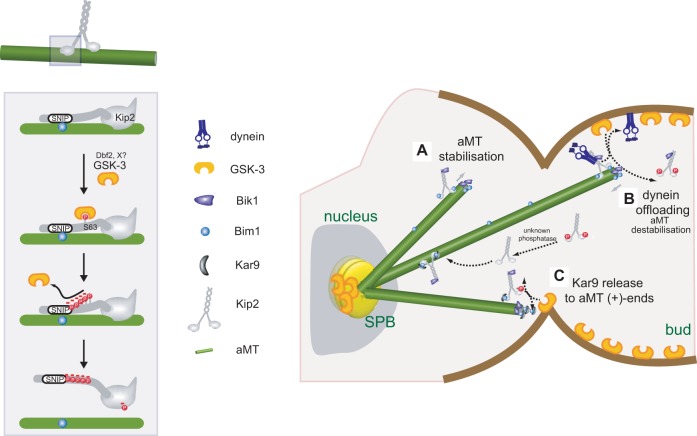
Fig. 7.
Model on how Mck1 regulates aMT functions. Left: Mck1 phosphorylates Kip2 at the N-terminal extension, including the GSK-3 consensus site that comprises S63, close to the EB1-binding motif (SNIP1). Phosphorylation disrupts interaction between Kip2 and Bim1. In addition, phosphorylation (involving possibly also T275) causes dissociation of Kip2 from aMTs. Dbf2 and unknown kinase(s) act as priming kinases for Mck1. Right: Mck1 might regulate aMT function through Kip2 phosphorylation in three ways. (A) Unphosphorylated Kip2 binds and stabilises aMTs. (B) When aMTs reach the cortex, Mck1 phosphorylates Kip2, leading to Kip2 dissociation from aMTs and offloading of dynein at cortical sites. The process may be enhanced upon anaphase onset, the time of Dbf2 activation. (C) Phosphorylation by Mck1 also disrupts interaction between Kip2 and Bim1, leading to release of transported Bim1–Kar9 complexes at aMT plus ends. Dephosphorylation of Kip2 by a phosphatase restarts the cycle.