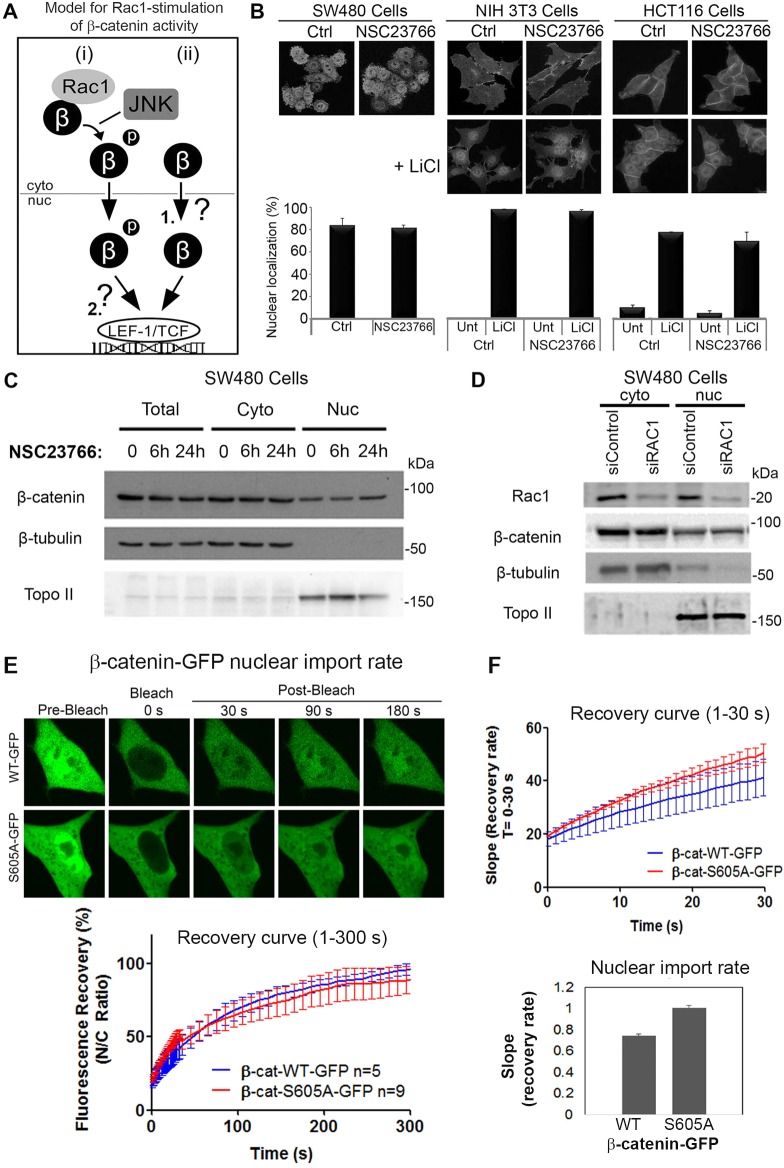
Fig. 2.
Blocking Rac1 activity or expression does not alter β-catenin nuclear localization. (A) Two likely mechanisms by which Rac1 can stimulate Wnt/β-catenin gene transactivation are shown: (i) Rac1-mediated phosphorylation of β-catenin drives nuclear import and accumulation of β-catenin (Wu et al., 2008; Phelps et al., 2009), and (ii) unphosphorylated β-catenin can enter the nucleus (1) and Rac1-mediated phosphorylation of β-catenin stabilizes its interaction with LEF-1 to drive transactivation (2). (B) To test the first model, different cell lines were treated for 6 h with 50 µM NSC23766 (Rac1 inhibitor) in the absence and presence of 40 mM LiCl. Cells were then fixed and stained for β-catenin, then analyzed by immunofluorescence microscopy. Representative cell images are shown. Two hundred cells were scored for nuclear localization of β-catenin and the data are plotted as means±s.d. from two independent experiments. The Rac1 inhibitor had no significant effect. (C) SW480 cells were treated with NSC23766 (50 µM) for 6 or 24 h, after which cells were lysed and β-catenin protein levels analyzed by western blotting. Blots were probed for anti-β-catenin, β-tubulin (cytoplasmic loading control; cyto) and Topoisomerase II (Topo II, nuclear loading control; nuc). (D) Rac1 knockdown did not affect β-catenin distribution. SW480 cells were transfected with Rac1 or control siRNAs for 72 h. Cells were then lysed and subjected to western blotting. Blots were probed for anti-Rac1, β-catenin, β-tubulin and Topo II. (E) pβ-catenin-WT-GFP and pβ-catenin-S605A-GFP were expressed in NIH3T3 cells and compared for nuclear import by FRAP. Nuclear fluorescence was bleached and recovery measured over 300 s (see cell images and recovery curve). (F) Analysis of the first 30 s post-bleach revealed that the S605A mutation did not inhibit nuclear import of β-catenin.