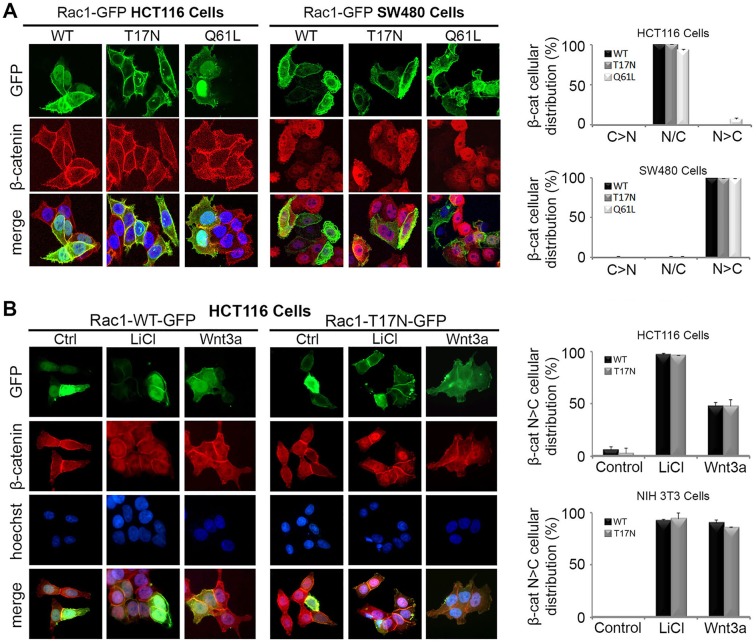
Fig. 3.
Overexpression of Rac1 does not affect nuclear localization of endogenous β-catenin in HCT116, SW480 or NIH 3T3 cells. (A) HCT116 and SW480 colorectal cancer cells were transfected with plasmids for GFP-tagged WT (wild-type), T17N (dominant negative) or Q61L (constitutively active) Rac1. Immunostaining with β-catenin antibody and microscopy of cells revealed that endogenous β-catenin (red) remains nuclear/cytoplasmic (N/C) in distribution regardless of Rac1-GFP (green) localization. The cytoplasmic (C) and nuclear (N) distribution of β-catenin is shown in the bar graphs (C>N, N/C, N>C) for each Rac1 construct. (B) Next, a dominant negative mutant form of Rac1 (T17N) was tested for its ability to block β-catenin nuclear accumulation in Wnt-stimulated HCT116 and NIH 3T3 cells. Cells were transfected with pRac1-WT-GFP or pRac1-T17N-GFP, treated with 40 mM LiCl or Wnt3a conditioned media for 6 h and then stained for GFP (green), β-catenin (red) and DNA (blue). Typical microscopy images of transfected HCT116 cells are shown. Column graph displays the cellular distribution (N>C) of endogenous β-catenin in Rac1-WT or T17N transfected cells in the absence and presence of LiCl/Wnt3a in HCT116 and NIH 3T3 cells.