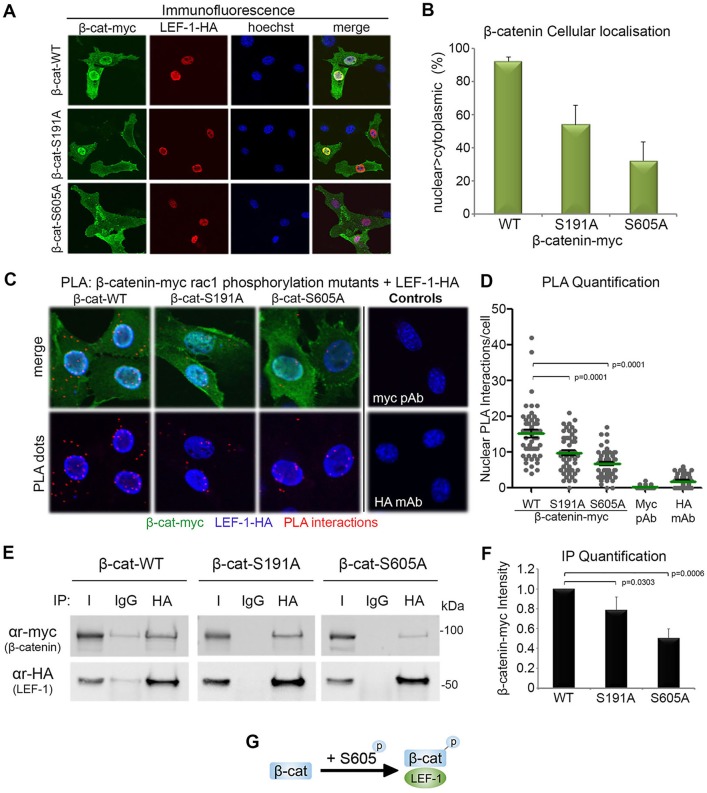
Fig. 7.
Mutation of the Rac1-regulated phosphorylation sites of β-catenin reduce its binding to LEF-1. Overexpression of pHA-LEF-1 anchors β-catenin to chromatin and stabilizes it in the nucleus of NIH 3T3 cells (Henderson et al., 2002). (A) We used this ability of LEF-1 to anchor β-catenin in the nucleus to first compare LEF-1 binding efficiency of wild-type and Rac1-regulatory site mutants (S191 and S605) of β-catenin. NIH 3T3 cells were co-transfected with plasmids encoding myc-tagged WT, S191A or S605A β-catenin and pHA-LEF-1 before being fixed and stained for α-myc (green: β-catenin), α-HA (red: LEF-1) and DNA (blue: Hoechst dye,). LEF-1 is seen to drag WT-β-catenin into the nuclei of cells more efficiently than it does for β-catenin-S191A and S605A mutants. (B) Cells were scored for nuclear>cytoplasmic staining of ectopic β-catenin in the LEF-1-transfected cells. (C) NIH 3T3 cells were co-transfected with myc-tagged β-catenin constructs (WT, S191A or S605A) and pHA-LEF-1 and subjected to PLA. α-myc and α-HA antibodies were used to detect the interaction between β-catenin and LEF-1. A significant reduction in nuclear interactions between the β-catenin mutants (S191A and S605A) and LEF-1-HA can be seen. (D) A dot plot shows the number of nuclear interactions between β-catenin and LEF-1 counted per cell. One hundred nuclei over two independent experiments were scored from z-stack confocal microscopy images and a representative graph is shown. (E) pLEF-1-HA was co-transfected with pβ-catenin-myc constructs (WT, S191A or S605A) in NIH 3T3 cells. Cell lysates were subjected to immunoprecipitation using HA mAb or isotype control IgG antibody. The blot was probed with anti-HA and myc antibodies and shows a reduction in β-catenin-phosphorylation mutant binding to LEF-1 compared with WT-β-catenin. (F) Column graph showing significant reduction in β-catenin mutant band intensity compared with wild-type analyzed by densitometry over three independent experiments. (G) Schematic diagram showing the phosphorylation of β-catenin at S605 (Rac1 mediated) increases the interaction between β-catenin and LEF-1.