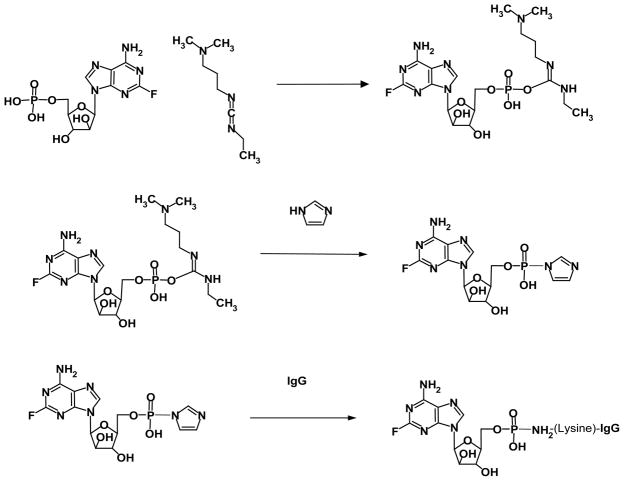
Figure 1.
Organic Chemistry Reaction Scheme for Synthesis of Covalent Fludarabine-(C2-methylhydroxyphosphoramide)-[anti-IGF-1R] Immunochemotherapeutic. Phase-I Reaction Scheme (Row Plate#1): reaction of the fludarabine C2-mono-phosphate group with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide to transiently form a reactive fludarabine carbodiimide phosphate ester intermediate complex; Phase-II Reaction Scheme (Row Plate II): rapid spontaneous conversion of the transient Phase I reactive intermediate to the Phase-II fludarabine-phosphorylimidazolide amine-reactive intermediate in the presence of imidazole. Phase-IIl Reaction Scheme (Row Plate III): covalent phosphoramide bond formation between the Phase-ll fludarabine-phosphorylimidazolide amine-reactive intermediate and μ-mononamine of lysine residues within the amino acid sequence of anti-IGF-1R monoclonal immunoglobulin resulting in the synthesis of a covalent fludarabine-(C2-methylhydroxyphosphoramide)-[anti-IGF-1R] immunochemotherapeutic.