Figure 3.
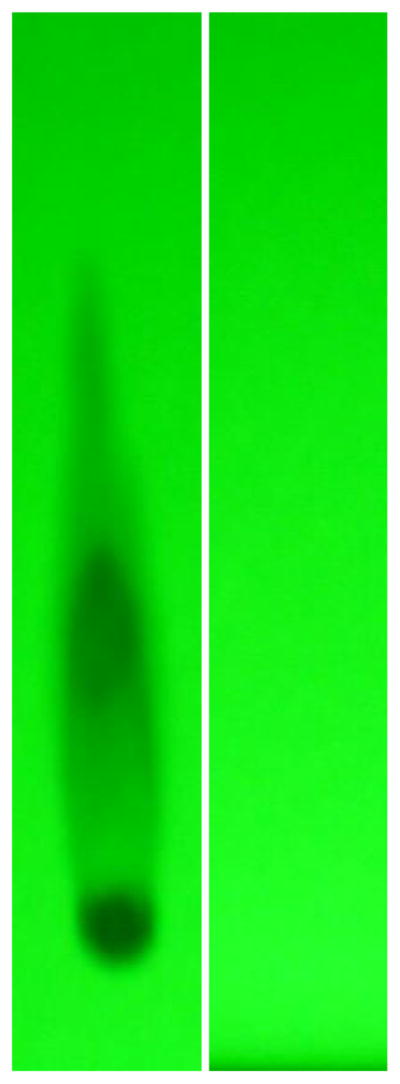
Evaluation of fludarabine-(C2-methylhydroxyphosphoramide)-[anti-IGF-1R] by analytical HP-TLC for the detection of residual fludarabine not covalently bound to anti-IGF-1R immunoglobulin. Legends : (Lane-1) Phase-II fludarabine-phosphorylimidazolide amine-reactive intermediate; and (Lane-2) Phase-III covalent fludarabine-(C2-methylhydroxyphosphoramide)-[anti-IGF-1R] immunochemotherapeutic following serial micro-filtration (MWCO = 10-kDa). Standardized fludarabine-equivalent concentrations of fludarabine-(C2-methylhydroxyphosphoramide)-[anti-IGF-1R] and the fludarabine-phosphorylimidazolide amine-reactive intermediate were applied to HP-TLC plates (silica gel, 250 μm thickness, UV 254 nm indicator) and developed utilizing a propanol/ ethanol/H20 (17:5:5 v/v) mobile phase. Identification of any residual fludarabine or un-reacted fludarabine-phosphorylimidazolide in the Phase-III covalent fludarabine-(C2-methylhydroxyphosphoramide)-[anti-IGF-1R] immunochemotherapeutic was subsequently determined by direct illumination with UV light.
