FIG. 1.
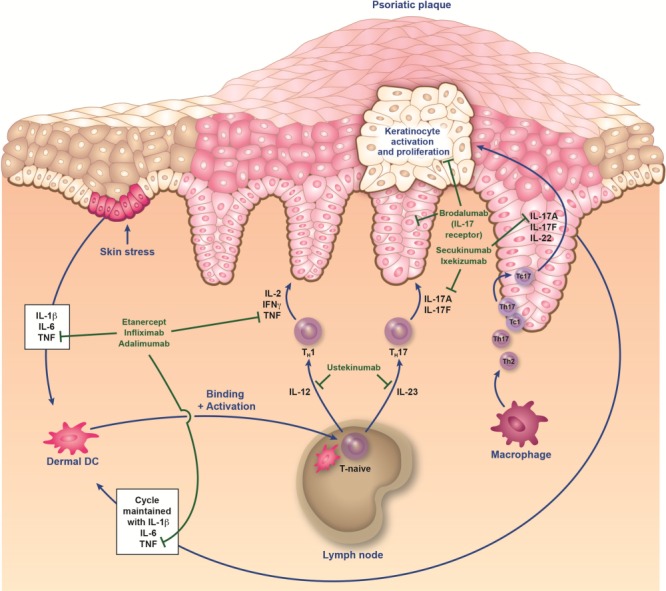
Immunopathogenesis of psoriasis and sites of action of biologics. It is proposed that an initial trigger (e.g., injury, infection, stress, etc.) precipitates a cascade of events that starts with the activation of innate immune cells (plasmacytoid dendritic cells, natural killer cells, and keratinocytes). These activated cells secrete cytokines (e.g., interferon [IFN] α, tumor necrosis factor [TNF] α, interleukin [IL]-1β, and IL-6), which in turn activate myeloid dendritic cells (DC). DC are central to the immune system, providing a link between innate and adaptive responses. The activated myeloid DC enter the draining lymph nodes, causing naive T cells (T-naive) to differentiate into helper type 17 (TH17) and type 1 (TH1) cells via the presentation of antigens and secretion of IL-12 and IL-23. These effector T cells then migrate into skin tissue, where they secrete mediators (eg, IL-17A, IL-17F, and IL-22 from TH17/cytotoxic T17 [TC17] cells, and IFNγ and TNFα from TH1/cytotoxic T1 [TC1] cells) that stimulate keratinocyte activation and proliferation, leading to plaque formation (12,13,20). Activated keratinocytes produce antimicrobial peptides, proinflammatory cytokines (IL-1β, TNF, and IL-6), and various chemokines that feedback into the proinflammatory cycle. This feedback loop, as well as others involving fibroblasts and endothelial cells, results in the continued immunopathologic progression of psoriasis.
