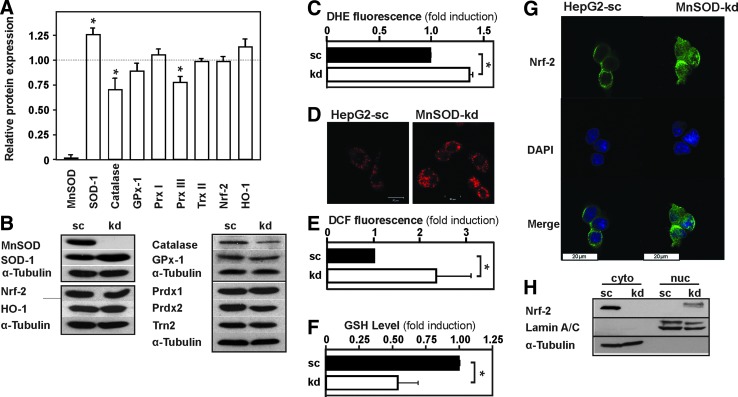
FIG. 1.
Knockdown of MnSOD induces ROS levels. (A) HepG2 cells were stably transfected with a scrambled (sc) shRNA or shRNA against MnSOD (kd). Protein lysates were prepared and analyzed by Western blotting with antibodies against MnSOD, Sod-1, Catalase, GPx-1, Prdx1, Prdx3, Trn2, Nrf-2, HO-1, and α-tubulin. Data are mean ± SD of fold induction normalized to HepG2-sc cells (n = 3, *p < 0.05) (B) Representative Western blots. α-tubulin is shown for equal loading of proteins. (C–E) The redox state of HepG2-sc and MnSOD-kd cells was determined by measuring (C) DHE fluorescence (n = 3), (D) DHE fluorescence microscopy showing mitochondrial localization of superoxide (n = 3), (E) DCF fluorescence (n = 10), and (F) GSH levels (n = 3) (c.f. section “Materials and Methods”). Data are mean ± SD of fold induction normalized to HepG2-sc (*p < 0.05). (G) Nrf2 localization analyzed by confocal fluorescence immunohistochemistry. (H) Representative Western blot showing Nrf2 localization after cell fractionation; Lamin A/C is shown to indicate nuclear fractions, and α-tubulin is shown for cytosolic fraction. DCF, dichlorodihydrofluorescein; DHE, dihydroethidium; Gpx-1, glutathione peroxidase-1; GSH, reduced glutathionel; HO-1, heme oxygenase-1; kd, knockdown; MnSOD, manganese superoxide dismutase; Nrf2, NF-E2-related factor 2; Prdx, peroxiredoxin; ROS, reactive oxygen species; SD, standard deviation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars